Despite the lack of solid evidence on their efficacy, hydroxychloroquine (HCQ) and azithromycin (AZ) have been widely used as a first-line treatment for infection with SARS-CoV-2, the causative agent of coronavirus disease 2019 (COVID-19). The effect of these drugs on the QT interval and their potential to cause polymorphic ventricular arrythmias has generated growing concern in the scientific community and until more robust evidence on their usefulness is available, we must employ strategies to ensure their safe use.1 Recently, the Food and Drug Administration recommended the use of noninvasive remote monitoring devices to facilitate the monitoring of these patients, which minimizes contact with health care professionals, reduces the burden on health care services and allows more efficient use of resources.2 To this end, the KardiaMobile 6L device, from AliveCor (California, USA), has been proposed, which can provide a 1- or 6-lead electrocardiogram (ECG), offering a simple and reproducible way to determine the corrected QT interval (QTc).3 Here in Spain, there are already protocols to support its use in these patients.4
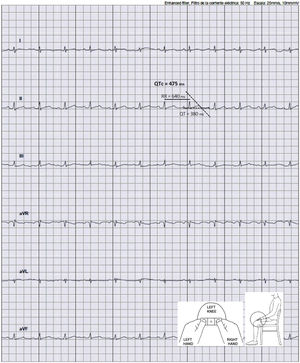
During March and April of 2020, a study was conducted in our hospital to analyze the effect of treatment with HCQ (either alone or in combination with AZ) on the QTc and the incidence of ventricular arrhythmias in patients admitted with SARS-CoV-2 pneumonia who met the high-risk criteria for QTc prolongation (female, age>65 years, history of heart disease, chronic renal disease, or diabetes, or taking both medications together). In line with the recommendations from the experts,3 a protocol was designed to minimize the arrhythmic complications of these drugs. This protocol included a series of precautions to be taken before and during treatment: a) review what other medications the patient is taking that could prolong the QTc; b) correct electrolyte imbalances; c) avoid bradycardia; and d) perform close electrocardiographic monitoring. A baseline 12-lead ECG was performed on admission. Later, the QTc was monitored using a 6-lead recording taken with the KardiaMobile 6L device, at 48hours and 96hours after starting the drugs (or more often if the QTc was>480ms, if there was an increase>60ms, or if the patient had possible symptoms of arrythmia). The arrhythmia unit trained the nursing staff responsible for these patients using an informational video on the use of KardiaMobile 6L. After a brief explanation from the nursing staff, the patient performed the recording, positioning the device on the left knee or ankle (as shown in figure 1). From outside the room, the nurse recorded the ECG on a tablet and transferred it to the electronic medical records. Four electrophysiologists analyzed the recordings and noted in the medical records the details of the ECG, the QTc measurement, and the recommendations on its treatment if they considered it necessary. For patients who were unable to perform the recording themselves, or when the tracing did not allow accurate measurement of the QTc, a 12-lead ECG was performed.
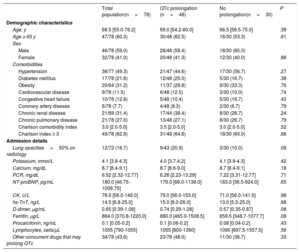
Of 306 patients admitted with COVID-19 pneumonia, 186 received HCQ and met criteria to be considered high risk. Of these, 78 were included in the electrocardiographic monitoring protocol (52 were taking the HCQ plus AZ combination). The baseline QTc was 425 (400-447) ms. Of all the patients, 48 (61.5%) had QTc prolongation on subsequent ECG; 18 (37.5%) were taking HCQ alone and 30 (62.5%) were taking HCQ plus AZ. The baseline characteristics of the study population are shown in table 1, as well as the comparison between the groups with and without prolonged QTc.
General characteristics of the patients admitted with COVID-19 who underwent electrocardiographic monitoring with the KardiaMobile 6L device (n=78)
| Total population(n=78) | QTc prolongation (n=48) | No prolongation(n=30) | P | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, y | 68.5 [55.0-78.2] | 69.0 [54.2-80.0] | 66.5 [56.5-75.0] | .39 |
| Age ≥ 65 y | 47/78 (60.3) | 30/48 (62.5) | 16/30 (53.3) | .61 |
| Sex | ||||
| Male | 46/78 (59.0) | 28/48 (58.4) | 18/30 (60.0) | |
| Female | 32/78 (41.0) | 20/48 (41.3) | 12/30 (40.0) | .88 |
| Comorbidities | ||||
| Hypertension | 38/77 (49.3) | 21/47 (44.6) | 17/30 (56.7) | .27 |
| Diabetes mellitus | 17/78 (21.8) | 12/48 (25.0) | 5/30 (16.7) | .38 |
| Obesity | 20/64 (31.2) | 11/37 (29.8) | 9/30 (33.3) | .76 |
| Cardiovascular disease | 9/78 (11.5) | 6/48 (12.5) | 3/30 (10.0) | .74 |
| Congestive heart failure | 10/78 (12.8) | 5/48 (10.4) | 5/30 (16.7) | .43 |
| Coronary artery disease | 6/78 (7.7) | 4/48 (8.3) | 2/30 (6.7) | .79 |
| Chronic renal disease | 21/69 (31.4) | 17/44 (38.4) | 8/30 (26.7) | .24 |
| Chronic pulmonary disease | 21/78 (27.0) | 13/48 (27.1) | 8/30 (26.7) | .79 |
| Charlson comorbidity index | 3.0 [2.0-5.0] | 3.5 [2.0-5.0] | 3.0 [2.0-5.0] | .52 |
| Charlson index ≥ 3 | 49/78 (62.8) | 31/48 (64.6) | 18/30 (60.0) | .68 |
| Admission details | ||||
| Lung opacities>50% on radiology | 12/72 (16.7) | 9/43 (20.9) | 3/30 (10.0) | .08 |
| Potassium, mmol/L | 4.1 [3.8-4.3] | 4.0 [3.7-4.2] | 4.1 [3.9-4.3] | .42 |
| Calcium, mg/dL | 8.7 [8.4-9.1] | 8.7 [8.6-9.0] | 8.7 [8.4-9.1] | .18 |
| PCR, mg/dL | 6.52 [2.32-12.77] | 6.26 [2.23-13.29] | 7.22 [3.31-12.77] | .71 |
| NT-proBNP, pg/mL | 180.0 [46.75-1009.75] | 176.0 [66.0-1138.0] | 183.0 [36.5-924.0] | .65 |
| CK, U/L | 78.0 [56.0-148.0] | 79.0 [56.0-153.0] | 71.0 [56.0-141.5] | .98 |
| hs-TnT, ng/L | 14.5 [6.8-25.0] | 15.0 [8.0-26.0] | 13.0 [5.5-25.0] | .68 |
| D-dimer, μg/mL | 0.65 [0.39-1.08] | 0.74 [0.29-1.28] | 0.57 [0.35-0.87] | .08 |
| Ferritin, μg/L | 864.0 [370.8-1220.0] | 880.0 [465.0-1508.5] | 859.5 [348.7-1077.7] | .08 |
| Procalcitonin, ng/mL | 0.1 [0.05-0.2] | 0.1 [0.06-0.2] | 0.08 [0.04-0.2] | .43 |
| Lymphocytes, cells/μL | 1055 [790-1055] | 1055 [800-1390] | 1090 [697.5-1557.5] | .56 |
| Other concurrent drugs that may prolong QTc | 34/78 (43.6) | 23/78 (48.0) | 11/30 (36.7) | .33 |
CK, creatine kinase; hs-TnT, high-sensitivity troponin T; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCR, polymerase chain reaction.
Values are expressed as n/N (%) or median [range].
No significant differences were found between the patients with and without prolonged QTc. The median QTc prolongation was 25.5 (16.8-57.5) ms, with no significant differences between the groups who were taking HCQ plus AZ and HCQ alone (p=0.38). The median QTc duration on monitoring was 450 (436-462) ms. Ten patients (12.8%) had a significant prolongation of the QTc (increase ≥ 60ms or QTc ≥ 500ms): 4 (5.1%) were taking HCQ alone and 6 (7.7%) were taking HCQ plus AZ. Five (6.4%) of these patients stopped the medication for this reason. We did not observe sustained ventricular arrhythmia or death due to arrhythmia in any of our patients during the monitoring period. Twelve patients (15.4%) required a conventional ECG due to difficulty interpreting the recording or not being able to use the device due to limited mobility.
According to our experience, HCQ and AZ treatment in patients admitted with COVID-19 is safe as long as measures are taken to minimize the risk of arrhythmia, including close electrocardiographic monitoring. Therefore, given the high burden on the healthcare system caused by this disease, and given its high rate of transmissibility, we think that ECG recording by the patients themselves with devices such as the KardiaMobile 6L can be a simple, useful strategy to avoid unwanted proarrhythmic effects of this treatment.
This study would not have been possible without the valuable contribution of all the health care professionals involved in the care of our patients with COVID-19. The authors would like to give special thanks to José Luis Ibáñez Criado, Thomas Brouzet, Esperanza Merino de Lucas, Isabel Lillo Ródenas, Vicente Arrarte Esteban, as well as the nurses involved in obtaining the recordings: Eunice González Ríos, Cristina Sogorb Garri, Regina S. Cardoso Monteiro and Norma Pérez Carpio.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.08.020