The COVID-19 pandemic poses an epidemiological challenge and a problem for diagnostic and therapeutic decision-making. The drugs used in patients infected with SARS-CoV-2 have an arrhythmogenic risk associated with QT prolongation, even in patients with a previously normal QT.1 Recent registries have confirmed that treatment with hydroxychloroquine (HCQ), whether in monotherapy or in combination with azithromycin, is associated with significant QT prolongation in COVID-19 patients.1–3 However, except in a few reported cases, its association with arrhythmia-related mortality is unclear, and some studies have noted a net neutral effect of these drugs on in-hospital mortality during the treatment of COVID-19 pneumonia,4 indicating the need for further studies of the arrhythmogenic risk of the treatments used.
Our objective was to determine the changes in QT interval from admission and their relationship with the combinations of drugs used in the treatment of COVID-19 pneumonia. We analyzed 1-month survival according to the degree of QT interval prolongation in the first 48hours of hospitalization.
We retrospectively included all patients admitted to our center with COVID-19 pneumonia at the start of the pandemic (March 2020) and with available electrocardiogram (ECG) data at baseline and at 48hours after treatment initiation (performed per protocol in our center). All ECGs were stored in digital format. The Bazett-corrected QT interval (QTc) was measured automatically (DXL ECG Algorithm, TMV, Philips, The Netherlands) and confirmed by 2 independent cardiologists if there was doubt. The at-risk group was defined as patients with long QTc > 460ms or with a QTc increase (iQTc) > 60ms. Notably, other studies have defined patients with a QTc > 500ms as being high risk and have recommended precaution with a QTc > 460 to 480ms.4 We selected a less restrictive cutoff point to permit a stricter follow-up of the at-risk group in order to determine the arrhythmic behavior of patients with COVID-19.
In total, 226 patients were included between March 1 and March 20, 2020. Recruitment was halted when the number of daily admissions impeded an exhaustive follow-up. Finally, 65 patients were excluded due to the lack of a digitalized baseline or 48-hour ECG, leaving 161 patients for the statistical analysis. The most frequently used specific therapeutic regimen was dual therapy with HCQ and lopinavir/ritonavir (LPV/r) (n = 111; 68.9%), followed by triple therapy with HCQ, LPV/r, and azithromycin or a quinolone (n = 30; 18.6%). Monotherapy with azithromycin or LPV/r was the least commonly used strategy (n = 12; 7.5%). Eight patients (5.0%) received alternative combinations.
The drug dosages were similar to those used in other centers. HCQ was administered at 400 mg/12 h in the first 24hours, followed by 200mg/12h. The LPV/r dosage was 400+100 mg/12 h, whereas that of azithromycin was 500 mg/24 h. Categorical variables were compared using the chi-square test and continuous variables using the t test. The survival analysis was an actuarial analysis using the Wilcoxon-Gehan test.
In general, the QTc interval was significantly higher at 48hours that at admission (443 ± 30 vs 435 ± 25ms; P = .001), with a mean iQTc of 8 ± 28 ms. No significant differences were found in the iQTc or in the QTc at 48hours among the different drug combinations.
The use of nonspecific drugs for SARS-CoV-2 significantly increased from 22.4% at admission to 37.9% at 48hours (P = .003) and the most frequently added drugs were metoclopramide, ondansetron, loperamide, and levofloxacin. Patients administered these drugs tended to show a higher iQTc than patients who were not administered them, which was almost significant (13 ± 28 vs 4 ± 28 ms; P = .066).
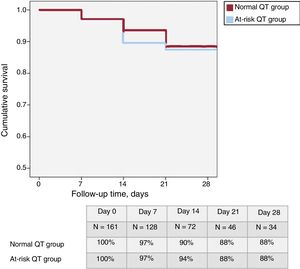
In total, 37 patients (23%) were classified in the “at-risk QT group”. Table 1 shows the baseline characteristics of this group, which were similar to those of the remaining patients. A total of 19 patients (12%) died during hospitalization, none due to arrhythmia. Only 7 patients had a QTc > 500 ms at 48 hours and their 1-month survival was not significantly different (86%; P = .187). At 1-month follow-up (figure 1), survival was similar in the at-risk patients and in those without QT prolongation (88% in both groups; P = .882). There was no difference in the need for intensive care unit admission (23 patients in the normal QT group and 7 in the at-risk group; P = .92).
Baseline characteristics of the study population
| All (n = 161) | Normal QT group (n = 124) | At-risk QT group (n = 37) | P | |
|---|---|---|---|---|
| Male sex | 103 (64) | 80 (49.7) | 23 (14.3) | .899 |
| Age, y | 63.9 ± 14.8 | 63.6 ± 15.6 | 64.7 ± 12.1 | .709 |
| Hypertension | 71 (44.1) | 52 (32.3) | 19 (11.8) | .279 |
| Diabetes mellitus | 25 (15.5) | 17 (10.6) | 8 (5) | .235 |
| Dyslipidemia | 57 (35.4) | 47 (29.2) | 10 (6.2) | .214 |
| Obesity (BMI > 30) | 42 (26.1) | 33 (20.5) | 9 (5.6) | .785 |
| COPD/asthma | 26 (16.2) | 19 (11.8) | 7 (4.4) | .591 |
| Heart disease | 26 (16.2) | 18 (11.2) | 8 (5) | .293 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease.
The results of this series are in line with those of previous series and show QT interval prolongation in patients with COVID-19 pneumonia treated with HCQ and azithromycin. In contrast to other series, most of our patients received LPV/r. No significant differences were detected in the QT interval prolongation among the different combinations of specific drugs. Attention must be paid not only to the drugs used to treat the infection, but also to other drugs administered during admission that can significantly prolong the QT interval. QT interval prolongation was not associated with higher mortality during the first month of follow-up and no arrhythmia-related mortality was detected.