SARS-CoV infection requires virus binding to the membrane-bound form of angiotensin-converting enzyme 2 (ACE2). Hydroxychloroquine (HCQ) inhibits terminal glycosylation of ACE2 receptor, which may reduce the efficiency of its interaction with SARS-CoV spike protein.1 Initial experiences during the COVID-19 pandemic supported the offlabel use of HCQ. However, its potential cardiotoxicity and still unclear benefit have eventually urged caution.2
High fatality rates have been reported in elderly individuals with COVID-19 and multiple cardiovascular comorbidities.3 Concerns have arisen that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) might increase ACE2 receptor expression and patient susceptibility to viral entry into host cells, facilitating SARS-CoV-2 propagation.4 Recent studies with different designs found no adverse effects associated with ACEIs/ARBs in various large populations with COVID-19 but did not report on HCQ coadministration.5
We analyzed 1031 patients admitted to the Hospital of Cremona, the epicenter of the COVID-19 outbreak in Italy, between February 22 and April 7, 2020, and followed up until May 3, 2020.
COVID-19 pneumonia was confirmed by chest computed tomography and a SARS-CoV-2-positive real-time reverse transcriptase-polymerase chain reaction assay from nasopharyngeal swabs. Treatment protocols were based on offlabel use of HCQ (400mg twice a day on the first day and 200mg twice a day thereafter for 10 days), as well as lopinavir/ritonavir or darunavir/ritonavir, intravenous methylprednisolone, empirical antimicrobial therapy, low-molecular-weight heparin, and supplemental oxygen.
From the hospital data warehouse, we extracted data on the admitting ward, cardiovascular risk factors and disease, drug therapies, and in-hospital outcomes. Demographic covariates (age, sex), cardiovascular covariates (smoking, hypertension, obesity, diabetes, atrial fibrillation, coronary heart disease, cerebrovascular disease, systolic dysfunction), and treatment covariates (antidiabetic agents, beta-blockers, calcium channel blockers, loop diuretics, antivirals, steroids) were tested by univariable Cox regression and those significantly associated (P <.10) with death or intensive care unit admission (combined end point) were entered in a multivariable model. Additionally, we performed weighted Cox regression using inverse probability of treatment weighted estimation with robust standard errors. A multivariable logistic regression model that included the same covariates as Cox regression was used to estimate the inverse probability of treatment weights for the individual propensities for ACEI/ARB receipt.
The institutional review board approved this retrospective analysis and waived the need for individual informed consent.
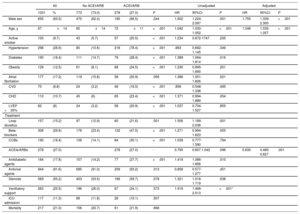
All 1031 patients received HCQ during hospitalization (table 1). Overall, 559 patients (54.2%) took ≥ 1 cardiovascular drugs (diuretics, beta-blockers, calcium channel blockers, or ACEIs/ARBs); of these, 278 (27%) received either an ACEI (135 [13.1%], 11±4mg/d enalapril equivalents) or an ARB (143 [13.9%], 64±34mg/d losartan equivalents) and 239 (86%) continued to take them throughout the hospitalization. Although patients treated with ACEIs/ARBs were older, had a higher cardiovascular comorbidity burden, and were more often taking antidiabetic agents and subject to cardiovascular polypharmacy, they had similar intensive care unit admission and mortality rates to patients not being treated with ACEIs/ARBs (table 1).
Characteristics of the study cohort and associations with the combined end point (death or intensive care unit admission) by Cox regression analysis
| All | No ACEI/ARB | ACEI/ARB | Unadjusted | Adjusted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1031 | % | 773 | (73.0) | 278 | (27.0) | P | HR | 95%CI | P | HR | 95%CI | P | |
| Male sex | 655 | (63.5) | 470 | (62.4) | 185 | (66.5) | .244 | 1.602 | 1.224-2.097 | .001 | 1.755 | 1.339-2.300 | <.001 |
| Age, y | 67 | ±14 | 65 | ±14 | 72 | ±11 | <.001 | 1.042 | 1.030-1.052 | <.001 | 1.046 | 1.035-1.057 | <.001 |
| Active smoker | 100 | (9.7) | 43 | (5.7) | 57 | (20.5) | <.001 | 1.234 | 0.872-1747 | .235 | |||
| Hypertension | 298 | (28.9) | 80 | (10.6) | 218 | (78.4) | <.001 | .883 | 0.682-1.145 | .349 | |||
| Diabetes | 190 | (18.4) | 111 | (14.7) | 79 | (28.4) | <.001 | 1.389 | 1.064-1.814 | .016 | |||
| Obesity | 129 | (12.5) | 61 | (8.1) | 68 | (24.5) | <.001 | 1.230 | 0.895-1.690 | .201 | |||
| Atrial fibrillation | 177 | (17.2) | 119 | (15.8) | 58 | (20.9) | .056 | 1.386 | 1.051-1.826 | .021 | |||
| CVD | 70 | (6.8) | 24 | (3.2) | 46 | (16.5) | <.001 | .856 | 0.548-1.338 | .495 | |||
| CHD | 110 | (10.7) | 45 | (6) | 65 | (23.4) | <.001 | 1.371 | 0.994-1.889 | .054 | |||
| LVEF <35% | 82 | (8) | 24 | (3.2) | 58 | (20.9) | <.001 | 1.037 | 0.704-1.527 | .855 | |||
| Treatment | |||||||||||||
| Loop diuretics | 157 | (15.2) | 97 | (12.9) | 60 | (21.6) | .001 | 1.556 | 1.189-2.038 | .001 | |||
| Beta-blockers | 308 | (29.9) | 176 | (23.4) | 132 | (47.5) | <.001 | 1.271 | 0.994-1.623 | .055 | |||
| CCBs | 190 | (18.4) | 106 | (14.1) | 84 | (30.1) | <.001 | 1.039 | 0.777-1.390 | .794 | |||
| ACEIs/ARBs | 278 | (27.0) | 278 | (27.0) | - | 0.795 | 0.607.1.042 | .096 | 0.630 | 0.480-0.827 | .001 | ||
| Antidiabetic agents | 184 | (17.8) | 107 | (14.2) | 77 | (27.7) | <.001 | 1.419 | 1.086-1.856 | .010 | |||
| Antiviral agents | 944 | (91.6) | 685 | (91.0) | 259 | (93.2) | .313 | 0.858 | 0.577-1.277 | .451 | |||
| Steroids | 569 | (55.2) | 403 | (53.5) | 166 | (59.7) | .078 | 1.321 | 1.016-1.719 | .038 | |||
| Ventilatory support | 263 | (25.5) | 196 | (26.0) | 67 | (24.1) | .573 | 1.919 | 1.466-2.513 | <.001* | |||
| ICU admission | 117 | (11.3) | 89 | (11.8) | 28 | (10.1) | .507 | ||||||
| Mortality | 217 | (21.0) | 156 | (20.7) | 61 | (21.9) | .668 | ||||||
95%CI, 95% confidence interval; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CCBs, calcium channel blockers; CHD, coronary heart disease; CVD, cerebrovascular disease; HR, hazard ratio; ICU, intensive care unit; LVEF, left ventricular ejection fraction.
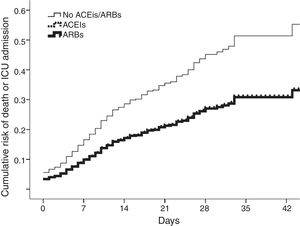
In total, 117 patients (11.3%) were admitted to the intensive care unit and 217 died (21%); 273 (27%) met the combined end point. After covariate adjustment (table 1), ACEIs/ARBs were independently associated with the combined outcome. ACEIs and ARBs conferred similarly lower risk (figure 1). The results were replicated in analysis restricted to mortality (adjusted hazard ratio [HR] for ACEIs/ARBs, 0.661; 95% confidence interval [95%CI], 0.490-0.890; P=.006) after further adjustment for the need for ventilatory support. The effects were consistent in the analysis weighted by inverse probability of treatment weighting (HR for ACEI/ARB use, 0.666; 95%CI, 0.445-0.997; P=.048).
Cumulative risk of death or intensive care unit (ICU) admission in patients on angiotensin-converting enzyme inhibitors (ACEIs) (dotted line), angiotensin receptor blockers (ARBs) (thick line), or neither (thin line). Curves for ACEIs and ARBs overlap, indicating comparably lower risk.
In our hospitalized cohort treated with HCQ for COVID-19 pneumonia, ACEIs/ARBs were independently associated with a lower hazard of mortality or severe disease requiring intensive care unit admission. ACEI or ARB receipt was balanced and both, administered at relatively high doses, had a similar impact on outcome. The findings were confirmed in the analysis weighted by inverse probability of treatment weighting.
Recent observational studies in geographically diverse populations found no differences in the need for invasive ventilation or death in patients with SARS-CoV-2 pneumonia treated with ACEIs/ARBs.5 None of these studies reported on the coadministration of HCQ, which might represent a confounding factor. Moreover, evidence on the benefits and harm of the use of HCQ or chloroquine to treat COVID-19 is still weak and conflicting.3,6
Our patients were older and had a higher burden of cardiovascular risk factors and comorbidities than previous series, all factors that may have contributed to high event rates. Intriguingly, during HCQ coadministration, ACEIs/ARBs were associated with lower hazards of mortality and the need for invasive ventilation, reinforcing previous findings of their lack of detriment in COVID-19.5
In a setting similar to ours, Geleris et al.6 found no association of HCQ treatment with a higher or lower risk of intubation or death. However, the study did not report the effects of ACEIs/ARBs, which were prescribed in rates similar to those of our cohort.
Our data do not allow confirmation of a possible synergy of ACEIs/ARBs and HCQ or a protective impact of ACEIs/ARBs on the potential cardiac adverse events of HCQ. In many cardiovascular diseases, ACEIs/ARBs exert beneficial effects on cardiac function and endothelial cell dysfunction, which might improve prognosis despite the use of cardiotoxic drugs.
The positive impact of ACEIs and ARBs in our aging patients with cardiovascular morbidities hospitalized for COVID-19, even during treatment with a potentially cardiotoxic agent, has particular relevance for cardiology practice and strengthens the recommendation to continue treatment with ACEIs/ARBs when indicated.
The authors gratefully acknowledge the contribution of Gaia Basini, MEng, to the data collection.