Coronavirus disease 2019 (COVID-19) seems to be associated with a higher risk of myocardial injury, especially in critically ill patients.1 Previous definitions of acute COVID-19 cardiovascular syndrome2 have been heterogeneous, and therefore its true incidence, clinical relevance and prognostic impact remain unclear. The aim of this study was to analyze echocardiographic abnormalities and biomarkers in COVID-19 patients requiring intensive care and their association with 30-day survival.
Observational, prospective cohort study of patients admitted to the intensive care unit (ICU) of Hospital Universitario La Paz (Madrid, Spain) with confirmed COVID-19 infection and acute respiratory distress syndrome between March 1 and April 8, 2020. We analyzed serum biomarkers in all patients. Following current recommendations,3,4 a focused cardiac ultrasound study3 was performed by accredited cardiologists. The main outcome was 30-day survival. Major cardiovascular events during follow-up were recorded, including myocarditis, pericarditis, pulmonary embolism (PE), and ventricular arrhythmias. The patients were followed up until hospital discharge or death. The study was conducted in compliance with the Declaration of Helsinki and was approved by the ethics committee of our institution.
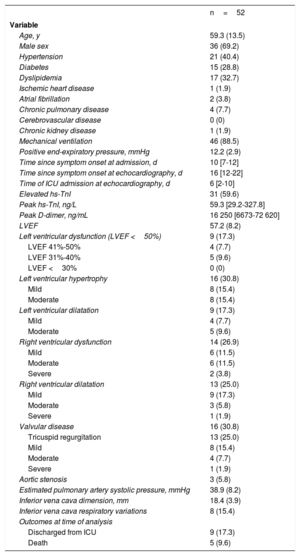
Fifty-two patients were included (table 1), and the median follow-up was 46 [22-54] days. The most common findings in our study were right ventricular (RV) abnormalities, mainly RV systolic dysfunction (26.9%) and dilatation (25.0%). Left ventricular systolic dysfunction and dilatation were less prevalent in our cohort.
Patient characteristics
| n=52 | |
|---|---|
| Variable | |
| Age, y | 59.3 (13.5) |
| Male sex | 36 (69.2) |
| Hypertension | 21 (40.4) |
| Diabetes | 15 (28.8) |
| Dyslipidemia | 17 (32.7) |
| Ischemic heart disease | 1 (1.9) |
| Atrial fibrillation | 2 (3.8) |
| Chronic pulmonary disease | 4 (7.7) |
| Cerebrovascular disease | 0 (0) |
| Chronic kidney disease | 1 (1.9) |
| Mechanical ventilation | 46 (88.5) |
| Positive end-expiratory pressure, mmHg | 12.2 (2.9) |
| Time since symptom onset at admission, d | 10 [7-12] |
| Time since symptom onset at echocardiography, d | 16 [12-22] |
| Time of ICU admission at echocardiography, d | 6 [2-10] |
| Elevated hs-TnI | 31 (59.6) |
| Peak hs-TnI, ng/L | 59.3 [29.2-327.8] |
| Peak D-dimer, ng/mL | 16 250 [6673-72 620] |
| LVEF | 57.2 (8.2) |
| Left ventricular dysfunction (LVEF <50%) | 9 (17.3) |
| LVEF 41%-50% | 4 (7.7) |
| LVEF 31%-40% | 5 (9.6) |
| LVEF <30% | 0 (0) |
| Left ventricular hypertrophy | 16 (30.8) |
| Mild | 8 (15.4) |
| Moderate | 8 (15.4) |
| Left ventricular dilatation | 9 (17.3) |
| Mild | 4 (7.7) |
| Moderate | 5 (9.6) |
| Right ventricular dysfunction | 14 (26.9) |
| Mild | 6 (11.5) |
| Moderate | 6 (11.5) |
| Severe | 2 (3.8) |
| Right ventricular dilatation | 13 (25.0) |
| Mild | 9 (17.3) |
| Moderate | 3 (5.8) |
| Severe | 1 (1.9) |
| Valvular disease | 16 (30.8) |
| Tricuspid regurgitation | 13 (25.0) |
| Mild | 8 (15.4) |
| Moderate | 4 (7.7) |
| Severe | 1 (1.9) |
| Aortic stenosis | 3 (5.8) |
| Estimated pulmonary artery systolic pressure, mmHg | 38.9 (8.2) |
| Inferior vena cava dimension, mm | 18.4 (3.9) |
| Inferior vena cava respiratory variations | 8 (15.4) |
| Outcomes at time of analysis | |
| Discharged from ICU | 9 (17.3) |
| Death | 5 (9.6) |
ICU, intensive care unit; hs-TnI, high-sensitivity troponin I; LVEF, left ventricular ejection fraction.
Data are expressed as No. (%), mean±standard deviation, or median [nterquartile range].
Median [interquartile range] high-sensitivity troponin I (hs-TnI) and peak D-dimer values are shown in table 1. D-dimer was significantly higher in patients with RV systolic dilatation (69 645 [36 621-122 040] vs 11344 [6519-52 363] ng/mL; P=.02) and dysfunction (63 872 [25 284-112 085] vs 10 972 [6498-59 450] ng/mL; P=.01) compared with those with a normal RV. In contrast, median hs-TnI was not associated with a higher incidence of cardiac structural abnormalities. No differences were found between positive end-expiratory pressure and RV function or dimension.
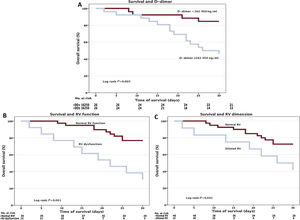
Kaplan-Meier survival analyses were performed for all patients. Survival at 30 days was significantly lower in patients with higher D-dimer values than the median (figure 1). Lower 30-day survival rates were also found in patients with RV dysfunction and dilatation.
Survival analyses. Kaplan-Meier survival curves representing cumulative survival at 30 days by A: peak D-dimer values (considering a median value of 16 250 ng/mL); B: right ventricular function; and C: right ventricular dimension. RV, right ventricular; RVF, right ventricular function; DD, D-dimer; No., number.
A multivariate Cox-proportional hazards regression analysis was performed including age, D-dimer values, and RV dysfunction. RV function was identified as an independent predictor of 30-day survival in our cohort (hazard ratio, 3.71; 95% confidence interval, 1.28-10-76; P=.02).
The major cardiovascular events rate was relatively low in our cohort. PE occurred in 3 patients, who required reperfusion therapy; only 1 was confirmed by catheter pulmonary angiography, showing multiple thrombi in the distal vessels. Findings compatible with pericarditis were present in 2 patients and 3 showed data consistent with myocarditis. No ventricular arrhythmias were identified in our cohort.
RV dysfunction and dilatation are common echocardiographic findings in ICU patients and are associated with worse outcomes. In addition, higher D-dimer levels suggest a prothrombotic state and correlate with RV abnormalities and worse survival. An isolated rise in hs-TnI does not seem to be associated with structural or clinically relevant cardiac abnormalities in COVID-19 patients.
These findings may be related to an increased prevalence of PE in COVID-19 patients. Nevertheless, in the ICU scenario, patients with acute respiratory distress syndrome requiring mechanical ventilation may also develop acute cor pulmonale, which may be indistinguishable. Surprisingly, the high positive end-expiratory pressure levels shown in our population did not correlate with RV abnormalities. Therefore, a hypothesis of a pulmonary prothrombotic state impacting on the RV should be raised and might be related to worse outcomes. Our data are consistent with autopsy reports5 and computed tomography scans6 of COVID-19 patients, showing RV dilatation and multiple thrombi in small pulmonary vessels.
Because our study is a single-center experience with a small sample size, it has some limitations. The main limitation is that we did not perform computed tomography due to the patients’ critical status and isolation protocols. Further research is needed to confirm our findings.
We thank Drs. M.J. Asensio Martín, J.M. Añón Elizalde, S.M. Sánchez Sánchez, and A. García de Lorenzo for their contribution to this article.