Despite the progress made over the past decades in the management of infective endocarditis, the morality rate is still high.1 Embolic events (EE) are the most frequent extracardiac complication, with an incidence of symptomatic EE ranging from 10% to 50%,2 affecting mainly the central nervous system and therefore worsening prognosis.3
The possibility of EEs also affects treatment options, as surgery is indicated to prevent them; a recent study showed that early surgery significantly reduces EE occurrence.4 As they are early-onset phenomena in infective endocarditis, it is essential to quantify the embolic risk at the time of diagnosis in order to avoid this complication and help in the therapeutic decision-making process.
Two systems for predicting embolic risk in infective endocarditis were designed recently, one French5 and one Italian6. Our objective was to test and compare the clinical utility of both systems.
We studied 153 consecutive patients admitted to a tertiary care center between January 2009 and April 2014 with a diagnosis of infective endocarditis according to the modified Duke criteria. In the first 24hours of admission, all patients underwent a transthoracic echocardiogram and 90% took a transesophageal one.
The embolic risk was calculated using the French system, which gives 1 point to each of the following covariates: diabetes mellitus, atrial fibrillation, vegetation > 10 mm, embolism prior to antibiotic therapy and Staphylococcus aureus. Age was added as a continuous variable to the sum of the above points, as per the French system, to estimate EE risk.5 The risk was also calculated using the Italian system, assigning 1 point to vegetation ≥ 13 mm and 1 point to S. aureus. Patients were classified as being at high risk of embolism if they had a probability > 7.5% according to the French system and of 2 points using the Italian system.
The performance of both systems in predicting EEs based on diagnosis and having started antibiotic therapy was evaluated using a Cox regression model. Embolic events based only on clinical suspicion or cutaneous manifestations were not considered.
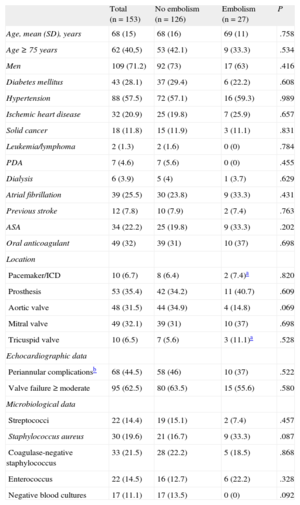
The clinical, echocardiographic, and microbiological data are shown in the Table. The Charlson comorbidity index was 4 (SD,2). The length of the vegetation was 8 (SD,7) mm on the transthoracic and 10 (SD,7) mm on the transesophageal echocardiogram. While in hospital, 27 (17.6%) EEs were recorded, 16 (59.9%) affecting the central nervous system. In-hospital mortality was 3 times higher (12/27; 44.4%) in patients with EE than in patients without EE (11.9%).
Baseline Characteristics
| Total (n = 153) | No embolism (n = 126) | Embolism (n = 27) | P | |
| Age, mean (SD), years | 68 (15) | 68 (16) | 69 (11) | .758 |
| Age ≥ 75 years | 62 (40,5) | 53 (42.1) | 9 (33.3) | .534 |
| Men | 109 (71.2) | 92 (73) | 17 (63) | .416 |
| Diabetes mellitus | 43 (28.1) | 37 (29.4) | 6 (22.2) | .608 |
| Hypertension | 88 (57.5) | 72 (57.1) | 16 (59.3) | .989 |
| Ischemic heart disease | 32 (20.9) | 25 (19.8) | 7 (25.9) | .657 |
| Solid cancer | 18 (11.8) | 15 (11.9) | 3 (11.1) | .831 |
| Leukemia/lymphoma | 2 (1.3) | 2 (1.6) | 0 (0) | .784 |
| PDA | 7 (4.6) | 7 (5.6) | 0 (0) | .455 |
| Dialysis | 6 (3.9) | 5 (4) | 1 (3.7) | .629 |
| Atrial fibrillation | 39 (25.5) | 30 (23.8) | 9 (33.3) | .431 |
| Previous stroke | 12 (7.8) | 10 (7.9) | 2 (7.4) | .763 |
| ASA | 34 (22.2) | 25 (19.8) | 9 (33.3) | .202 |
| Oral anticoagulant | 49 (32) | 39 (31) | 10 (37) | .698 |
| Location | ||||
| Pacemaker/ICD | 10 (6.7) | 8 (6.4) | 2 (7.4)a | .820 |
| Prosthesis | 53 (35.4) | 42 (34.2) | 11 (40.7) | .609 |
| Aortic valve | 48 (31.5) | 44 (34.9) | 4 (14.8) | .069 |
| Mitral valve | 49 (32.1) | 39 (31) | 10 (37) | .698 |
| Tricuspid valve | 10 (6.5) | 7 (5.6) | 3 (11.1)a | .528 |
| Echocardiographic data | ||||
| Periannular complicationsb | 68 (44.5) | 58 (46) | 10 (37) | .522 |
| Valve failure ≥ moderate | 95 (62.5) | 80 (63.5) | 15 (55.6) | .580 |
| Microbiological data | ||||
| Streptococci | 22 (14.4) | 19 (15.1) | 2 (7.4) | .457 |
| Staphylococcus aureus | 30 (19.6) | 21 (16.7) | 9 (33.3) | .087 |
| Coagulase-negative staphylococcus | 33 (21.5) | 28 (22.2) | 5 (18.5) | .868 |
| Enterococcus | 22 (14.5) | 16 (12.7) | 6 (22.2) | .328 |
| Negative blood cultures | 17 (11.1) | 17 (13.5) | 0 (0) | .092 |
ASA, acetylsalicylic acid; PDA, parenteral drug addict; ICD, implantable cardioverter-defibrillator; SD, standard deviation.
The estimations generated with the French system showed a significant association with embolic risk (hazard ratio [HR] = 2.7; 95% confidence interval [95%CI], 1.37-5.46). However, of the 27 patients who suffered an EE, 12 (44.4%) had been classified as low risk. The occurrence of EE was better predicted by this system than by chance (P = .03), although its discrimination was moderate (C-statistic = 0.66; 95%CI, 0.54-0.77).
The estimations from the Italian model were also associated with embolic risk: HR = 2.2 (95%CI, 1.28-3.92) and C = 0.62 (95%CI, 0.49-0.74; P= .05). In this model, 85.2% (n = 23) of patients who suffered an EE had been classified as low risk.
Calibration of both models was adequate (P≥.2). Compared with the Italian system, the French one showed a net reclassification improvement index of 7.4% (P = .5).
Therefore, although both models have a similar predictive power, the French system better classifies patients who are at low embolic risk than the Italian one. The main advantage of the Italian system is that it is easier to use, requiring only two variables, whereas the French system, apart from using a greater number of variables, requires software for its calculation5. In our cohort, only 1 patient had a right-sided embolism. Excluding her from the analysis did not affect the general results; therefore, in essence our results refer to left-sided embolisms.
In conclusion, in this contemporaneous series of infective endocarditis, approximately 1 in 6 patients had EE after their diagnosis or start of antibiotic therapy. The unadjusted excess risk of in-hospital death of patients with EE is around 30%. It is possible to predict the embolic risk with a simple clinical tool. In our population, the French system was shown to be more useful; therefore, including it as part of the clinical judgement should help to improve the therapeutic decision-making process, making it easier and more personalized. This would make it possible to reduce the rate of devastating complications, such as cerebral septic embolism. A more precise clinical tool than those currently available should nonetheless be developed.