Recent advances in the treatment of patients with severe aortic stenosis have led to greater use of unconventional procedures as an alternative to classic aortic valve replacement.1,2 One of these procedures is sutureless prosthesis implantation. These prostheses reduce the aortic cross-clamp and extracorporeal circulation time, and are particularly useful for high-surgical-risk patients and for minimally-invasive approaches.3,4
There is previous experience in the treatment of conventional bioprosthesis dysfunction using valve-in-valve percutaneous procedures,5 but such experience is rare in the treatment of sutureless bioprosthesis dysfunction.
We report the case of a 79-year-old woman with hypertension and dyslipidemia, who underwent valve replacement in 2011 due to severe degenerative aortic stenosis by using a Perceval size S sutureless prosthesis (Sorin Group; Saluggia, Italy) with an aortic cross-clamp time of 23minutes and extracorporeal circulation time of 45minutes. This is a self-expandable bovine pericardial prosthesis fixed in a Nitinol frame without the need for sutures, leaving the leaflets in the annular position and the fixation structure in the supra-annular position. The outcome was good, with no periprosthetic regurgitation and with a maximum transvalvular gradient of 23 mmHg, no perioperative complications, and an excellent clinical course. At discharge, the valve area was 1.6 cm2, and the indexed valve area was 1cm2/m2.
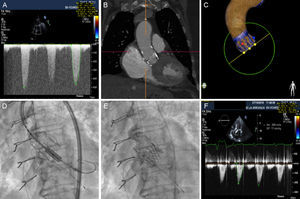
The patient remained asymptomatic during the follow-up period, but developed permanent atrial fibrillation in 2015 and consequently began oral anticoagulation with acenocoumarol. The patient reported the gradual onset of dyspnea from early 2016. A transthoracic echocardiogram revealed thickened, calcified leaflets, with restricted opening leading to severe aortic stenosis (peak gradient [PG], 99mmHg; mean gradient [MG], 49mmHg), with a valve area of < 0.5cm2 (Figure A, Video 1 of the supplementary material). The patient had an international normalized ratio (INR) of 2 to 3 in all determinations over the last 6 months. Given evidence of valve degeneration on ultrasound and adequate anticoagulation, the case was assessed by the local multidisciplinary team, composed of a clinical cardiologist, an interventional cardiologist, a cardiac surgeon, and a geriatrician. The patient was considered to be at high risk for conventional surgery, given her frailty (logistic EuroSCORE, 18%; Society of Thoracic Surgeons score, 4.335%). Despite the presence of a small prosthesis, it was decided to perform a percutaneous procedure, given that the prosthetic valve was sutureless.
A: transvalvular gradient of the degenerated prosthesis. B: computed tomography image of the Perceval prosthesis. C: Heart Navigator image for planning the procedure. D: alignment of the SAPIEN 3 prosthesis during the valve-in-valve procedure. E: angiographic result, which revealed a position slightly below the edge of the Perceval prosthesis. F: optimal transvalvular gradient at discharge, after implantation.
The preliminary study was completed with computed tomography (Figure B) and a transesophageal echocardiogram to assess the optimal arterial access and the effective annular size. In accordance with the manufacturer's specifications and with the help of the ViV Aortic app 2.0 (UBQO Limited), a true internal lumen of 17.5-19mm was established, which is compatible (among others) with a 23-mm SAPIEN 3 prosthesis (Edwards Lifesciences, Inc; Irvine, California, United States).
The implantation was carried out with conscious sedation and transthoracic echocardiogram monitoring. The Heart Navigator system was used as a reference to determine the optimal projection and confirm the size of the selected prosthesis (Figure C). Via right femoral access with surgical exposure, with a 14-Fr sheath, the e-sheath device was advanced over an extrastiff guidewire, according to a previously described procedure.6 In the alignment process, the radio-opaque marker of the SAPIEN 3 prosthesis was aligned with the lower edge of the Perceval prosthesis (Figure D). Once aligned, the prosthesis was implanted without predilation, with rapid ventricular stimulation. The hemodynamic outcome was optimal (Figure E), with a resulting maximum gradient of 20 mmHg and a mean gradient of 10 mmHg, with no significant regurgitation (Figure F, Video 2 of the supplementary material). Angiography showed that the prosthesis remained in a position slightly below the lower edge of the Perceval prosthesis. After implantation, the patient experienced complete atrioventricular block. A provisional pacemaker was implanted with implantation of a definitive VVIR device after 48hours. The patient progressed favorably and was discharged 5 days after implantation. Two months later, she remained asymptomatic.
This case is an example of the new reality in terms of aortic valve treatment and the emergence of new procedures. Multidisciplinary care in these cases allows us to provide patients with an enhanced level of safety. This is one of the few published cases that demonstrates the safety of transfemoral aortic prosthesis valve-in-valve implantation over a previous sutureless prosthesis.