Over the past few years, transcatheter aortic valve implantation has become increasingly consolidated as an alternative to aortic valve replacement surgery for patients who are at high surgical risk or are inoperable.1
The second generation of transcatheter aortic valve prostheses2–4 has recently become commercially available. These devices are designed to minimize major limitations such as paravalvular aortic regurgitation, the need for a pacemaker, or the malapposition associated with the first 2 transcatheter aortic valve prostheses: Edwards-SAPIEN and CoreValve (Medtronic; Minneapolis, Minnesota, United States).5,6 The new, self-expanding aortic prosthesis with the Medtronic CoreValve EvolutTM R 23mm system for aortic annuli < 20mm has been available in Spain since September 2014. The objective of this study was to analyze the initial experience with the first patients treated with the EvolutTM R and describe the major differences with respect to the first generation of CoreValve prostheses.
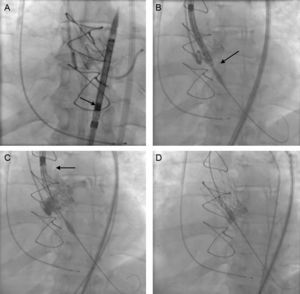
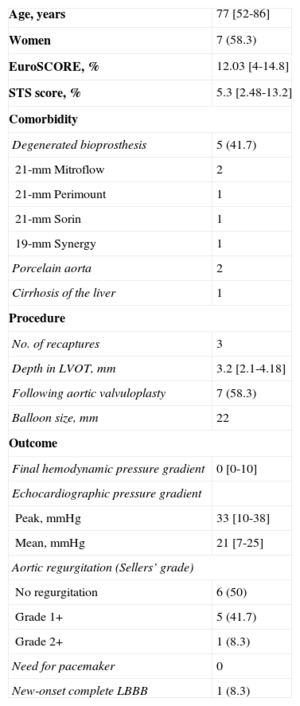
Continuous variables are presented as the median [interquartile range] and categorical variables as number (%). A total of 12 patients were treated between September 2014 and January 2015. The baseline characteristics of the patient population and the procedure are shown in the Table. All the procedures were carried out under local anesthesia and deep sedation, except in 1 patient in whom the approach was via the left subclavian artery. Notably, 5 patients had dysfunctional aortic valve bioprostheses. The diameter of the aortic valve annulus ranged from 18mm to 20mm. One new aspect of this valve is that, once the prosthesis is mounted on the delivery system, it is imperative that it be inspected under fluoroscopy, especially to ensure that the capsule containing the prosthesis shows no deformity or curvature and that the crowns are aligned and the anchoring tabs perfectly inserted and symmetric (Figure A).
Clinical Characteristics and Procedure Used With the CoreValve EvolutTM R 23mm System (n=12)
| Age, years | 77 [52-86] |
| Women | 7 (58.3) |
| EuroSCORE, % | 12.03 [4-14.8] |
| STS score, % | 5.3 [2.48-13.2] |
| Comorbidity | |
| Degenerated bioprosthesis | 5 (41.7) |
| 21-mm Mitroflow | 2 |
| 21-mm Perimount | 1 |
| 21-mm Sorin | 1 |
| 19-mm Synergy | 1 |
| Porcelain aorta | 2 |
| Cirrhosis of the liver | 1 |
| Procedure | |
| No. of recaptures | 3 |
| Depth in LVOT, mm | 3.2 [2.1-4.18] |
| Following aortic valvuloplasty | 7 (58.3) |
| Balloon size, mm | 22 |
| Outcome | |
| Final hemodynamic pressure gradient | 0 [0-10] |
| Echocardiographic pressure gradient | |
| Peak, mmHg | 33 [10-38] |
| Mean, mmHg | 21 [7-25] |
| Aortic regurgitation (Sellers’ grade) | |
| No regurgitation | 6 (50) |
| Grade 1+ | 5 (41.7) |
| Grade 2+ | 1 (8.3) |
| Need for pacemaker | 0 |
| New-onset complete LBBB | 1 (8.3) |
LBBB, left bundle branch block; LVOT, left ventricular outflow tract.
Unless otherwise indicated, the values are expressed as no. (%) or median [interquartile range].
Implantation of an aortic self-expanding prosthesis over a dysfunctional Perimount bioprosthesis, with the Medtronic CoreValve EvolutTM R 23mm system. A: Fluoroscopic assessment of the correct mounting of the prosthesis. B: The implantation target was 3±2mm. C: The tactile recapture point is found when 80% of the prosthesis is deployed. D: Complete deployment of the prosthesis.
The delivery system enables the use of a 14F introducer sheath, which was used in 6 patients with borderline femoral artery diameters; the usual 18F introducer sheath was used in the remainder.
Another of the changes observed is the implantation target. The previous recommendation was for the target to range between 4mm and 6mm with respect to the aortic annulus; it should now be 3 ± 2mm, given the change in the configuration of the structure and prosthesis (Figure B). Once correct alignment with the valve annulus has been achieved, the prosthesis is deployed by means of a knob that is turned in counterclockwise direction; the transmission of this movement to the capsule housing the prosthesis is practically 1:1. When the first two thirds have unfolded, the new system presents what is known as a tactile recapture point (Figure C). At this time, fluoroscopy and aortography are used to confirm the position of the prosthesis and coronary patency. As the position was good in 9 patients, the deployment was completed (Figure D); however, in 3 patients, the position was too low and it was necessary to retrieve the prosthesis and begin the positioning and delivery again, without having to withdraw the prosthesis, by turning the knob in a clockwise direction. This maneuver can be carried out up to 3 times. The implantation was successful in all the patients and there were no procedure-related complications. Eleven patients were discharged from the hospital and 1 died of respiratory failure secondary to pneumoconiosis.
The new system presents a couple of modifications with respect to the first generation system. The major change is the possibility of recapturing the prosthesis (by enveloping it in the delivery system) once 70% to 80% of the prosthesis has been deployed, with the valve functioning, a feature that allows verification of its position with respect to the native valve and coronary artery flow. Once the valve has been recaptured, it can be retrieved through the annulus, and its delivery can be undertaken again. The second modification is the reduction in the size of the delivery system by 4F, which means that its use can be extended to more patients with borderline femoral access.
The possibility of recapturing and repositioning the prosthesis enables operators to be more exacting and self-assured during positioning with respect to the native aortic annulus and, thus, to avoid an implantation that is too deep, which is associated with paravalvular leak, and changes in atrioventricular conduction (factors related to mortality during follow-up), as well as too high a position or passage of the device into the aorta and the possibility of occlusion of the coronary arteries. Another potential advantage is a possible dramatic reduction in the need for a second valve, which is reported to be around 5% in many series.
Thus, these areas of improvement over the first generation of the CoreValve aortic prosthesis will help to minimize some of the complications of the procedure and obtain better results.
CONFLICTS OF INTERESTJ.H. Alonso-Briales, C. Morís, and J.M. Hernández García are proctors for Medtronic.