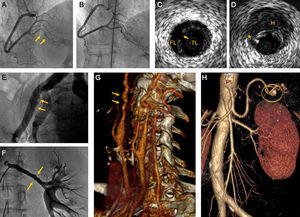
Spontaneous coronary artery dissection (SCAD) is a very uncommon clinical entity, whose etiology and pathophysiology are still not fully understood. The main diagnostic key of this entity is the finding-on coronary angiography-of a linear contrast defect, with longitudinal distribution (Figures A and B). Intracoronary imaging techniques (intravascular ultrasound and optical coherence tomography) allow more precise morphological diagnosis1 (Figures C and D).
A: Coronary angiography (patient 2) with spontaneous coronary artery dissection in the posterior descending artery (arrows). B: The same patient 6 months later, with complete resolution on angiography. C: Intravascular ultrasound in spontaneous coronary artery dissection (patient 1); true lumen and false lumen separated by an intimomedial flap, and the entry point (arrow). D: Intravascular ultrasound showing coronary hematoma (patient 1); a parietal hematoma (H) and guidewire artefact (*). E: External iliac artery (patient 9); angiographic irregularities compatible with fibromuscular dysplasia (arrows). F: Left renal artery (patient 7) with parietal irregularities typical of fibromuscular dysplasia (arrows). G: Computed tomography angiography of supra-aortic trunks (patient 5); Both internal carotid arteries have the characteristic string of beads appearance (arrows). H: Computed tomography angiography (patient 4) showing splenic artery aneurysm of 7mm diameter (circle). FL, false lumen; H, parietal hematoma; TL, true lumen.
SCAD has been associated with multiple diseases, and recently, with fibromuscular dysplasia (FMD), which is another rare arterial disease, neither inflammatory nor atherosclerotic, of equally uncertain etiology. In patients with SCAD, the systematic search for noncoronary arterial vascular disease has shown a prevalence of FMD of around 70%.2,3
In this study, we describe our experience with the diagnosis of FMD in patients with diagnosed SCAD.
From April 2011 until November 2014, patients diagnosed with SCAD in our center were included in a protocol of investigation and clinical follow-up (mean follow-up, 17 months). A “conservative strategy” was followed, which only indicated revascularization in patients with persistent symptoms or recurrent ischemia. The imaging technique used was specifically directed at the detection of arterial vascular disease in noncoronary territories.
Nine consecutive patients were included: 1 man and 8 women; all patients were middle-aged (Table). The diagnosis of SCAD was made during coronary angiography, which was done because of acute coronary syndrome. In 3 patients, diagnosis was made using intravascular ultrasound or optical coherence tomography.
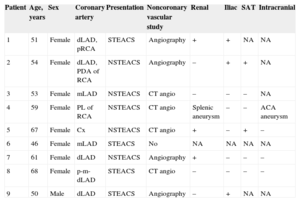
Patient Demographics, Clinical Features, and Results of Extracardiac Arterial Disease Study
| Patient | Age, years | Sex | Coronary artery | Presentation | Noncoronary vascular study | Renal | Iliac | SAT | Intracranial |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 51 | Female | dLAD, pRCA | STEACS | Angiography | + | + | NA | NA |
| 2 | 54 | Female | dLAD, PDA of RCA | NSTEACS | Angiography | – | + | + | NA |
| 3 | 53 | Female | mLAD | NSTEACS | CT angio | – | – | – | NA |
| 4 | 59 | Female | PL of RCA | NSTEACS | CT angio | Splenic aneurysm | – | – | ACA aneurysm |
| 5 | 67 | Female | Cx | NSTEACS | CT angio | + | – | + | – |
| 6 | 46 | Female | mLAD | STEACS | No | NA | NA | NA | NA |
| 7 | 61 | Female | dLAD | NSTEACS | Angiography | + | – | – | – |
| 8 | 68 | Female | p-m-dLAD | STEACS | CT angio | – | – | – | – |
| 9 | 50 | Male | dLAD | STEACS | Angiography | – | + | NA | NA |
ACA, anterior communicating artery; CT angio, computed tomography angiography; Cx, circumflex artery; LAD, left anterior descending artery (p, proximal; m, mid; d, distal); NA, study not available; NSTEACS, non-ST-elevation acute coronary syndrome; PDA, posterior descending artery; PL, posterolateral artery; pRCA, proximal right coronary artery; SAT, supra-aortic trunks; STEACS, ST-elevation acute coronary syndrome; +, pathological findings; –, no abnormal findings.
Angiography signifies selective injection into each renal artery (Judkins R4 catheter, 10mL each injection), aortography of aortic arch and supra-aortic trunks, and infrarenal aortography (pig tail catheter, 45mL each injection). CT angiography, Toshiba Aquilion 64 detector multislice computed tomography; 2 block helical study with single bolus contrast at a dose of 0.2mL/kg at a rate of 5mL/s; automatic exposure control, at a dose of 938 mGycm2.
Eight patients underwent extracardiac arterial disease study (1 patient declined further investigation). The arterial territories studied were the renal arteries, the iliofemoral arteries, the supra-aortic trunks, and the intracranial vessels (Table). In half the patients, invasive angiography was used (either during the index procedure or scheduled), and in the other half of the patients, computed tomography angiography was used. Of the 8 patients studied, only 1 had completely normal extracardiac arteries. In 5 patients, there were findings compatible with FMD, of variable severity (Figures E-G). One patient had a splenic artery aneurysm and an anterior communicating artery aneurysm in the circle of Willis (Figure H). The remaining patient, who was older, had multiple calcified plaques in the abdominal aorta and iliac arteries, of atherosclerotic etiology.
Until very recently, our knowledge of SCAD was based on isolated cases and short retrospective series. Nowadays, we have access to information from longer prospective series2–4: SCAD generally affects women of middle age, with not without classic cardiovascular risk factors and with no clear association with immunological, inflammatory, or connective tissue diseases, although isolated cases have been described in all those situations. Traditionally, SCAD has been associated with the final weeks of pregnancy and the postnatal period, but we now know that this association has a low prevalence.2–4 Also, recent studies indicate the benefit of an initial conservative strategy, reserving revascularization for patients with persistent or recurrent symptoms.4
Recently, several groups have highlighted the association of SCAD with FMD.2,3 The most common form of FMD is medial fibroplasia. The characteristic angiographic pattern of medial fibroplasia is that of alternating dilated and stenosed areas, forming what is called a “string of beads” appearance. Fibromuscular dysplasia can also be associated with aneurysms and dissections, which may form a pathophysiological link with SCAD. Anatomicopathologically, this image corresponds with alternating zones of thickening and thinning of the arterial medial layer. These findings have recently been visualized in vivo using optical coherence tomography.5,6
The high prevalence of the association of SCAD with FMD, 2 very uncommon diseases, raises suspicion of a strong pathophysiological relationship between them. The data that we present support this association (75% of the patients). In our experience, computed tomography angiography directed at detecting FMD has an adequate diagnostic value. It must be acknowledged that, in our series, the low number of patients hinders evaluation of the power of this association and its possible clinical implications. Also, the screening of extracardiac arterial disease was done with different techniques, which was a limitation of the study.
The clinical significance of extracardiac findings is very difficult to establish. Probably, mild parietal changes should only be considered “stigmata” of FMD, with no functional repercussions, and should not alter these patients’ management. However, more severe changes (significant aneurysms or stenoses), may require specific therapeutic interventions. The pathophysiological implications of this new association are not clear. New studies are needed with systematic screening for noncoronary vascular disease in patients with SCAD to establish the possible implications of this interesting association.