Percutaneous implantation of an aortic prosthesis in patients with severe aortic stenosis and high surgical risk has been proven to be more effective than medical treatment1 and similar or superior2 to conventional surgery according to the experience gained with the Edwards-SAPIEN (Edwards Lifesciences; Irvine, California, United States) and Medtronic CoreValve (Medtronic; Minneapolis, Minnesota, United States) prostheses in clinical trials1,2 and clinical practice registers.3
The DirectFlow4,5 prosthesis (DirectFlow Medical; Santa Rosa, California, United States) has recently been marketed; its potential advantages over existing devices are the possibility of repositioning/recapturing the device, hemodynamic stability during the procedure, and a low rate of significant aortic failure.
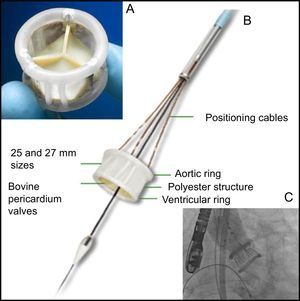
The DirectFlow prosthesis (Figure) does not have any metal components and its structure is a system of communicating vessels, which when filled with liquid, make 2 rings (fluid entry via the distal ring, exit via the proximal ring) connected by a polyester structure; the lower ring stabilises the prosthesis and the upper ring fixes the 3 bovine pericardium cusps. The deployment system comprises 3 cables that are attached to the lower ring and allow filling of the rings and positioning and deployment of the valve.
A: DirectFlow prosthesis seen from the aorta. B: Lateral view of the prosthesis and deployment system (positioning cables and internal lumen holding the support guide). C: Prosthesis in position with the 2 rings full of contrast medium; in this situation, the hemodynamic functionality and patency of the coronary arteries can be evaluated. At this time, it is still possible to reposition the prosthesis and recapture it if necessary.
The valve fitted in its sheath (compatible with an 18 Fr introducer) is advanced up to the left ventricle, where both rings are filled with a mixture of contrast medium and water. The upper ring is then emptied and the lower ring is positioned just below the valve with the help of the 3 traction cables. The upper ring is then filled and the position, functionality, and coronary permeability are assessed. If the position is not optimal, the upper ring is deflated, the lower ring repositioned, and the upper ring reinflated. The result is then re-evaluated. When this is satisfactory, the contrast medium is replaced by a polymer that solidifies in 10minutes and the fixation cables are released.
The selection criteria are: 20–26mm ring (for 25 and 27mm valves; size 23 is used for rings of 18mm or more), ring-coronary ostia distance >12mm and absence of massive calcification. Previous valvuloplasty is essential in all cases.
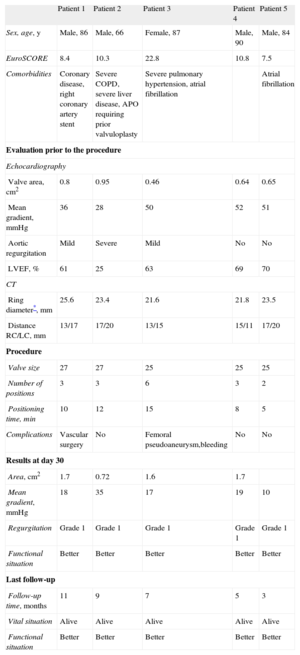
We report our initial experience with this valve. Twelve patients were evaluated, of whom 7 were considered ineligible (5 due to small ring size, 1 due to ring-coronary distance <12mm, and 1 for both reasons). Finally, 5 patients were treated (Table) in whom a valve was correctly implanted, with mild or trivial aortic regurgitation in all cases. The hemodynamic result was good in 4 patients, although the gradients were slightly higher than those recorded with other percutaneous valves, but similar to those reported with surgical biological prostheses6; in 1 patient with a functionally bicuspid valve due to fusion and calcification of the commissure between the right sinus and the noncoronary sinus, the initial postoperative gradient rose from 12 to 35mmHg by the third day and a geometrical deformity of the polyester structure was observed via computed tomography and fluoroscopy. Despite the residual gradient (stable since then), and probably due to a reduction in the degree of aortic failure (from grade 3 to grade 1), the patient improved clinically and still has good functional status. Both prosthetic function and clinical outcome were favourable in the other patients.
Baseline Data on the Procedure and Follow-up of the 5 Patients Treated
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
| Sex, age, y | Male, 86 | Male, 66 | Female, 87 | Male, 90 | Male, 84 |
| EuroSCORE | 8.4 | 10.3 | 22.8 | 10.8 | 7.5 |
| Comorbidities | Coronary disease, right coronary artery stent | Severe COPD, severe liver disease, APO requiring prior valvuloplasty | Severe pulmonary hypertension, atrial fibrillation | Atrial fibrillation | |
| Evaluation prior to the procedure | |||||
| Echocardiography | |||||
| Valve area, cm2 | 0.8 | 0.95 | 0.46 | 0.64 | 0.65 |
| Mean gradient, mmHg | 36 | 28 | 50 | 52 | 51 |
| Aortic regurgitation | Mild | Severe | Mild | No | No |
| LVEF, % | 61 | 25 | 63 | 69 | 70 |
| CT | |||||
| Ring diameter*, mm | 25.6 | 23.4 | 21.6 | 21.8 | 23.5 |
| Distance RC/LC, mm | 13/17 | 17/20 | 13/15 | 15/11 | 17/20 |
| Procedure | |||||
| Valve size | 27 | 27 | 25 | 25 | 25 |
| Number of positions | 3 | 3 | 6 | 3 | 2 |
| Positioning time, min | 10 | 12 | 15 | 8 | 5 |
| Complications | Vascular surgery | No | Femoral pseudoaneurysm,bleeding | No | No |
| Results at day 30 | |||||
| Area, cm2 | 1.7 | 0.72 | 1.6 | 1.7 | |
| Mean gradient, mmHg | 18 | 35 | 17 | 19 | 10 |
| Regurgitation | Grade 1 | Grade 1 | Grade 1 | Grade 1 | Grade 1 |
| Functional situation | Better | Better | Better | Better | Better |
| Last follow-up | |||||
| Follow-up time, months | 11 | 9 | 7 | 5 | 3 |
| Vital situation | Alive | Alive | Alive | Alive | Alive |
| Functional situation | Better | Better | Better | Better | Better |
RC, right coronary; LC, left coronary; APO, acute pulmonary oedema; COPD, chronic obstructive pulmonary disease; LVEF, left ventricle ejection fraction; CT, computed tomography.
The main advantages of the DirectFlow prosthesis over the Edwards-SAPIEN and Medtronic CoreValve prostheses are as follows:
- •
Greater flexibility
- •
Better hemodynamic stability during deployment (valve operative during implantation, does not require high-frequency stimulation)
- •
Possibility of evaluating the prosthesis prior to its deployment.
- •
Possibility of repositioning/recapturing the device.
- •
Low rate of aortic failure.
The disadvantages include:
- •
Less radial strength
- •
Slightly higher transvalvular gradient
The information available on this valve is still limited. The results presented at the 2014 EuroPCR congress seem to confirm the stability of the hemodynamic results after 1 year in the first 100 patients treated. Nonetheless, there are aspects, such as the impact of the residual gradient or the selection criteria, that require studies in larger samples and with longer follow-up periods. There are currently no data comparing the clinical results with those of the Edwards-SAPIEN and Medtronic CoreValve prostheses.