Currently, transcatheter aortic valve implantation is a well-established therapeutic option for the treatment of patients with inoperable symptomatic and severe aortic stenosis or with high risk for surgery.1 Although the midterm results in this type of patient are good, moderate-severe residual paravalvular aortic regurgitation (6%) or stroke (3%), and their prognostic implications, have been observed in recent years.2,3
Paravalvular aortic regurgitation can be caused by the implantation of a smaller-sized prosthesis, its inadequate expansion or intense calcification of the ring, which impedes correct stent apposition. The incidence of this complication has been reduced by more precise measurement of the aortic ring by using computed tomography and posterior balloon dilatation of the stent.4
At the same time, industry has developed second-generation valves that minimize the risk of residual paravalvular aortic regurgitation. Among them is the Lotus™ (Boston Scientific, Natick, Massachusetts, United States),5 which is a bovine pericardial tissue valve incorporated in a nitinol stent. The Lotus™ is preloaded and designed to provide more precise release and the possibility of its repositioning or recovery after implantation. This stent also minimizes the risk of paravalvular aortic regurgitation with its new-age sealing system (urethane membrane) that adapts to the irregular surface of the ring.
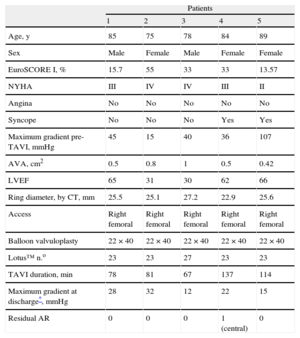
This article presents 5 consecutive cases of severe degenerative aortic stenosis treated with the Lotus™ aortic stent. Average patient age was 84 (SD, 5.63) years, and the EuroSCORE was 33% (SD, 16.7%) (Table).
Characteristics of the 5 Patients, Procedure and Results After Transcatheter Implantation of the Lotus™ Valve
| Patients | |||||
| 1 | 2 | 3 | 4 | 5 | |
| Age, y | 85 | 75 | 78 | 84 | 89 |
| Sex | Male | Female | Male | Female | Female |
| EuroSCORE I, % | 15.7 | 55 | 33 | 33 | 13.57 |
| NYHA | III | IV | IV | III | II |
| Angina | No | No | No | No | No |
| Syncope | No | No | No | Yes | Yes |
| Maximum gradient pre-TAVI, mmHg | 45 | 15 | 40 | 36 | 107 |
| AVA, cm2 | 0.5 | 0.8 | 1 | 0.5 | 0.42 |
| LVEF | 65 | 31 | 30 | 62 | 66 |
| Ring diameter, by CT, mm | 25.5 | 25.1 | 27.2 | 22.9 | 25.6 |
| Access | Right femoral | Right femoral | Right femoral | Right femoral | Right femoral |
| Balloon valvuloplasty | 22×40 | 22×40 | 22×40 | 22×40 | 22×40 |
| Lotus™ n.o | 23 | 23 | 27 | 23 | 23 |
| TAVI duration, min | 78 | 81 | 67 | 137 | 114 |
| Maximum gradient at discharge*, mmHg | 28 | 32 | 12 | 22 | 15 |
| Residual AR | 0 | 0 | 0 | 1 (central) | 0 |
AR, aortic regurgitation; AVA, aortic valve area; CT, computed tomography; LVEF, left ventricular ejection fraction; TAVI, transcatheter aortic valve implantation.
The severity of the stenosis was assessed by transthoracic echocardiography and a hemodynamics study with left-right catheterization. The indication for implantation was discussed and accepted in all patients except 1, who underwent urgent implantation due to hemodynamic instability.
The procedures were done under general anesthesia with transesophageal echocardiography. Before valvuloplasty, a provisional active-fixation pacemaker for ventricular overstimulation was implanted in the septum of all patients. Right femoral access was created in all patients; the puncture was guided by fluoroscopy and, after preparing the area with 2 ProGlides™ (Abbot Vascular, United States), the 18Fr sheath was introduced until the descending aorta. The native aortic valve was crossed over with the standard technique, taking special care not to pass the mitral subvalvular apparatus with the guide. Once the passage was confirmed by transesophageal echocardiography, a super-stiff Safari™ guide (0.035″, 260 cm) (Boston Scientific) was introduced into the left ventricle and valvuloplasty was done with a NuCLEUS™ 22×40mm balloon (NuMED Inc., Canada) in rapid stimulation at 180 bpm. Afterward, the Lotus™ valve was implanted in accordance with the recommended technique, without the need for overstimulation. Number 23 stents were implanted in 4 of the patients and a number 27 in 1. The size of the prosthesis was determined by the diameter of the area measured on computed tomography; in 2 patients, the final decision was supported by angiography at the time of the valvuloplasty. The implantation was successful in all patients, although 1 stent required repositioning. During implantation, the patients were hemodynamically stable at all times.
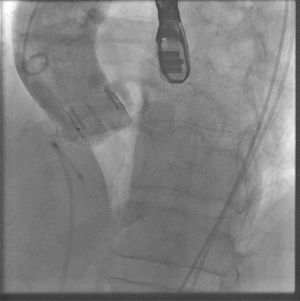
The proper function of the prosthesis was immediately checked after the intervention by measuring the transvalvular gradient with hemodynamic and transesophageal echocardiography. The absence of aortic insufficiency was verified with transesophageal echocardiography and aortography (in patients without moderate-severe renal failure) (Figure).
The temporary pacemaker was withdrawn after 48hours in all patients and the in-hospital clinical course transpired without incidences, with no need to implant permanent pacemakers and no appearance of clinical or clinically evident stroke. The patients were discharged from the hospital 4 (SD, 1.09) days after the procedure. On the TTE prior to discharge, we observed a mean maximum gradient of 21.8 (8.4) mmHg; only 1 patient had central mild aortic insufficiency (grade 1/4 by color Doppler), and none showed residual paravalvular aortic regurgitation.
A telephone follow-up was done 30 days after implantation. All patients reported functional class improvement: 3 were in functional class I/IV, and 2 in class II/IV. One of the patients was readmitted 28 days after the procedure due to chest pain, although the valve was observed to be functioning properly and there was no coronary arterial disease.
These are the first 5 patients treated with the Lotus™ transcatheter aortic valves reported in our country. We consider that the advances made in prostheses will enable us to achieve better results. The new sealing system for Lotus™ valves reduces residual paravalvular aortic regurgitation without the need to oversize the prosthesis relative to the ring, which could reduce complications such as ring rupture and thus allow expansion of the indications for transcatheter aortic valve implantation.