Interventional cardiologists have spent years working on and dreaming about transcatheter aortic valve implantation. In 2007-2008 we were privileged in Spain1,2 to use 2 of the most commonly-implanted devices at the time: the Cribier-Edwards aortic valve prosthesis1 and the CoreValve self-expanding prosthesis.2 These devices had successful initial results in high-surgical-risk patients with severe aortic stenosis. However, it soon became apparent that the devices had a number of limitations preventing their use in all patients with severe aortic stenosis. Furthermore, the implantation procedure sometimes led to complications and suboptimal results.3,4 After this initial experience, interventional cardiologists have now identified some areas where they would like to see technical improvements5:
- 1.
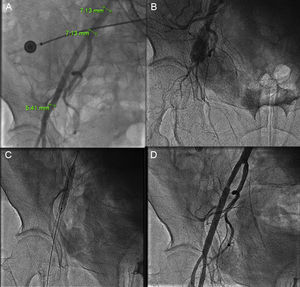
A lower profile device to permit delivery with introducers measuring less than 18 F. A transfemoral approach requires a vessel with a diameter of at least 6 mm (for an 18-F sheath). Femoral complications are fairly common6,7 (Figure 1) and are associated with the artery orifice size made during valve access. Also, patients with small femoral vessels or severe ileo-femoral occlusive disease are not candidates for transfemoral valve implantation.
Figure 1.A: Femoral rupture after a Prostar closure device failure in a femoral artery with a diameter of less than 6 mm. This complication occurred after a successful transcatheter aortic valve implantation using an 18-F introducer. B: Angiography showing vessel rupture. C: Implantation of 2 coated stents. D: Final result.
(0.23MB). - 2.
A wider range of prosthetic sizes to accommodate all annulus types from 17 mm to 29 mm. This need has gradually been met in recent years. Manufacturers have now introduced new models that cover virtually all aortic annulus sizes. The only exception are the newest prostheses on the market, but it is likely that they will also be available in a full range of sizes before long.
- 3.
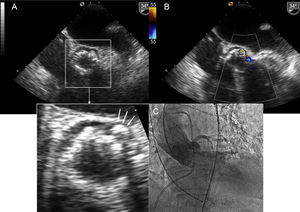
Improved prosthetic anchoring to the aortic annulus to reduce periprosthetic regurgitation, which is a key factor in the postimplantation clinical outcome.8 Irregular calcification on the native annulus sometimes prevents good apposition between the valve structure and the periprosthetic tissue, which in turn causes a varying degree of regurgitation, even if the valve is properly positioned (Figure 2). Therefore, next-generation transcatheter aortic valves should be designed to reduce paravalvular leakage.
- 4.
A device that is easy to reposition and completely retrievable if necessary. The valve must be correctly deployed in the aortic annulus. High deployment may cause aortic embolism, while low deployment may cause severe aortic regurgitation. To achieve good results immediately after the procedure, it is essential to select the appropriate site and be able to reposition the valve in an optimum location. Positioning can be challenging in aortas that lie on a very horizontal plane and in tortuous aortas.
- 5.
Specific accessories, such as introducers, guidewires and femoral closure devices, must be available. Transcatheter aortic valve implantation is a new technique and it therefore needs specialized instrumentation. Over the years, smaller-diameter, hydrophilic-coated guidewires have been brought out to improve transfemoral insertion. More recently, dedicated guidewires have been designed to pass the valve into the ventricle using a preshaped tip to reduce the risk of left ventricular perforation.9
- 6.
Long-term durability, ensuring that the system lasts for 10 to 15 years. More time is needed to show that transcatheter aortic valve implantation lasts as long as surgical valve replacement.
Initial experience with 3 new transcatheter aortic valves has been reviewed in 3 articles in Revista Española de Cardiología. The new devices are SAPIEN 3 (Edwards Lifesciencies; Irvine, California, USA),10 Lotus (Boston Scientific; Natick, Massachusetts, United States)11 and DirectFlow (DirectFlow Medical; Santa Rosa, California, United States).12 These devices have some of the technical improvements mentioned above.
Sapien 3The design of Sapien 3 differs significantly from earlier models made by the same manufacturer. Its delivery system is available with a 14-F sheath (for 23-mm and 26-mm valves) or a 16-F sheath (for 29 mm). Small sheath size has been achieved through the simplified cobalt chromium alloy frame, reduced strut thickness, and improved valve crimping system. It is the lowest profile delivery system currently available and it could become the system of choice for patients with small-diameter femoral vessels (Figure 1).
Sapien 3 also has a polyethylene terephthalate inner and outer skirt, designed to minimize residual paravalvular leak. This is particularly beneficial for patients with an asymmetrical calcified annulus (Figure 2). In fact, none of the patients described in the article10 had aortic regurgitation. The delivery system has a dual flex feature to improve valve positioning control and a radiopaque center marker on the balloon, which facilitates alignment along the valve plane. This feature is also an advantage for patients with a tortuous aorta.
LotusThis device is a bovine pericardial tissue valve mounted on a preloaded nitinol stent. It is delivered over an 18-F sheath. With regard to the technical improvements mentioned above, this valve can be repositioned or retrieved before final implantation. It is also useful in patients with a highly tortuous aorta, which poses a challenge to correct device positioning. Another interesting new technical feature is the seal system with a urethane membrane, designed to conform to irregular calcified annulus surfaces (Figure 2). This technique minimizes the risk of paravalvular aortic regurgitation. In the series published in Revista Española de Cardiología, none of the patients had this complication.11
DirectFlowThis prosthesis differs from the above-mentioned devices because it is a metal-free structure with communicating channels that are filled with a solution to conform 2 rings connected by a polyester frame. The lower ring anchors the prosthesis and the upper ring secures the 3 bovine pericardial leaflets. The delivery system consists of 3 wires attached to the lower ring. The positioning wires are used for ring filling and valve deployment. This device is also delivered over an 18-F sheath. It has 3 advantages over classical systems: it is retrievable, repositionable, and its ring inflation system creates a tight seal inside the periprosthetic tissue, thus minimizing the risk of periprosthetic regurgitation. As with the other devices, there were no reports of significant aortic regurgitation in the third case series.12One drawback is its reduced radial strength and slightly higher transvalvular pressure gradient, observed in 1 patient in the article.12
FUTURE PROSPECTSFuture indications for transcatheter aortic implantation will depend on the improvements over current devices in the coming years. We have mentioned many of these improvements above, and we expect to see a similar rate of change in the near future. Another key determinant in future of transcatheter aortic valves will be the findings of randomized studies comparing these valves with surgical valve replacement. In a recent study conducted in patients with severe aortic stenosis at high surgical risk, CoreValve implantation recipients had a higher 1-year survival rate than patients who underwent surgical valve replacement.13 In any event, more studies are required in patients at a lower surgical risk. To be able to expand current indications, we also need to know how long these prostheses will last in the long-term. At present, transcatheter aortic valve implantation is reserved for patients at high surgical risk. Since the technique was introduced, the number of implantations performed has risen notably, from 10000 implantations worldwide at the beginning of 2010, to more than 60000 in 2013 (data supplied by Medtronic, the manufacturer of CoreValve). This rate of growth is expected to continue in coming years. The technical improvements made to the new devices, as reflected in the articles published in Revista Española de Cardiología,10–12 doubtlessly contribute to the growth rate. If this process follows the same trend as percutaneous revascularization in patients with heart disease, it is likely that there will soon be more transcatheter aortic valve implantations than surgical valve replacements, as was the case with heart revascularization. Whatever the future holds for transcatheter aortic valve implantation in the coming years, cardiologists who treat patients with aortic stenosis must work in close collaboration with one another.
CONFLICTS OF INTERESTNone declared.