The various clinical guidelines and consensus statements recommend a multifactorial approach to diabetes mellitus, acting on glycemia and the other associated risk factors to obtain the greatest possible reduction in macrovascular and microvascular morbidity and mortality.1 Diabetic cardiomyopathy (DCM) is usually asymptomatic in the initial stages.2 Growth differentiation factor 15 (GDF-15) is a cytokine secreted by macrophages and cardiac myocytes in response to oxidative stress and inflammation.3
Our group recently described the usefulness of GDF-15 as a screening tool in the diagnosis of DCM in asymptomatic patients with type 2 diabetes mellitus.4 The study aimed to describe the prognostic value of GDF-15 at 1 year in a cohort of patients with DCM, evaluating the relationship between the levels of this biomarker and the combined primary endpoint of heart failure with hospital admission and/or angina with hospital admission.
The details of the study have previously been described.4 Briefly, the study prospectively included 213 asymptomatic patients with type 2 diabetes mellitus. Diabetic cardiomyopathy was defined, according to the criteria of the European Society of Cardiology and the European Association of Diabetes, as left ventricular diastolic dysfunction (tissue Doppler with an E/E¿ ratio ≥ 15) in the absence of hypertension, ischemic heart disease, or other structural heart disease.5 For the analysis, the outcomes at 365 days of the 45 patients who had DCM were studied, as was the relationship between DCM and GDF-15 concentration.
Continuous variables are expressed as mean±standard deviation for those with a normal distribution or median [interquartile range] for those with an abnormal distribution. Categorical variables are expressed as number (percentage). Quantitative variables were compared using the Student t test or Mann-Whitney U test. The association between qualitative variables was determined using the Pearson chi-square test or Fisher exact test. Survival analysis was performed using Cox regression, with variables with a P-value < .2 on univariate analysis being included in the model. Risk proportionality assumption was evaluated by Schoenfeld residual analysis. The statistical analysis was performed with the SPSS program, version 20 (SPSS Inc., Chicago, Illinois, United States).
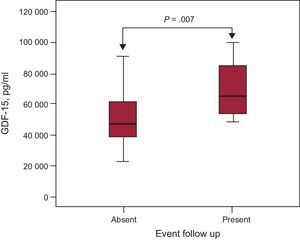
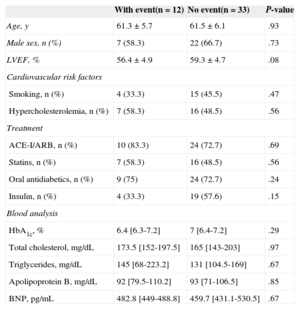
The study population characteristics are shown in the Table. The primary endpoint of the study occurred in 12 patients (26.7%). There were no statistically significant differences between the 2 groups in baseline characteristics (age, sex, left ventricular ejection fraction, hypercholesterolemia, and smoking), treatment, or laboratory data. Concentrations of GDF-15 were higher in patients with DCM who experienced a combined event than in those with no events (6458.9 pg/mL [5359.7-8681.9 pg/mL] vs 4706 pg/mL [3719-6463 pg/mL], P=.007) (Figure). Combined events occurred at a mean time of 162±89 days. In the Cox survival analysis, after adjustment for other covariables (treatment with insulin, mean GDF-15, and left ventricular ejection fraction), the variables shown to be independent predictors of the combined endpoint were mean GDF-15 level (hazard ratio=4.96; 95% confidence interval, 1.24-19.77; P=.023) and left ventricular ejection fraction (hazard ratio=0.83; 95% confidence interval, 0.7-0.98; P=.031). The discriminatory power of the model, determined using the C-statistic, was 0.826.
Classification of Patients With Diabetic Cardiomyopathy by Occurrence of Events
| With event(n= 12) | No event(n= 33) | P-value | |
|---|---|---|---|
| Age, y | 61.3± 5.7 | 61.5± 6.1 | .93 |
| Male sex, n (%) | 7 (58.3) | 22 (66.7) | .73 |
| LVEF, % | 56.4± 4.9 | 59.3± 4.7 | .08 |
| Cardiovascular risk factors | |||
| Smoking, n (%) | 4 (33.3) | 15 (45.5) | .47 |
| Hypercholesterolemia, n (%) | 7 (58.3) | 16 (48.5) | .56 |
| Treatment | |||
| ACE-I/ARB, n (%) | 10 (83.3) | 24 (72.7) | .69 |
| Statins, n (%) | 7 (58.3) | 16 (48.5) | .56 |
| Oral antidiabetics, n (%) | 9 (75) | 24 (72.7) | .24 |
| Insulin, n (%) | 4 (33.3) | 19 (57.6) | .15 |
| Blood analysis | |||
| HbA1c, % | 6.4 [6.3-7.2] | 7 [6.4-7.2] | .29 |
| Total cholesterol, mg/dL | 173.5 [152-197.5] | 165 [143-203] | .97 |
| Triglycerides, mg/dL | 145 [68-223.2] | 131 [104.5-169] | .67 |
| Apolipoprotein B, mg/dL | 92 [79.5-110.2] | 93 [71-106.5] | .85 |
| BNP, pg/mL | 482.8 [449-488.8] | 459.7 [431.1-530.5] | .67 |
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; DCM, diabetic cardiomyopathy; HbA1c, glycosylated hemoglobin; LVEF, left ventricular ejection fraction.
The originality of this study is that it demonstrates for the first time that high GDF-15 levels are associated with poor prognosis at 365 days in patients with DCM. Hyperglycemia activates signaling pathways mediated by reactive oxygen species, leading to the development of myocardial hypertrophy and fibrosis with ventricular stiffness and chamber dysfunction.2 Experimental studies have demonstrated that cardiac DGF-15 expression significantly increases after various forms of stress, including pressure overload.6 Given that GDF-15 is produced by several other types of cells besides cardiac myocytes (endothelial cells, adipocytes, and macrophages), it is likely that this biomarker comprises information from several disease pathways, providing the pathophysiological information necessary in patients with DCM.4
The main limitation of this study is its small sample size and low number of combined events and therefore it should be considered a hypothesis generator. We conclude that in patients with DCM, high GDF-15 values are associated with poor prognosis at 1 year. Randomized studies with larger sample sizes are needed to add more information on the value of this biomarker in predicting events.