The coronavirus disease of 2019 (COVID-19) is an infectious disease caused by severe acute respiratory coronavirus 2 (SARS-CoV-2).1 Most patients with severe disease develop pneumonia and acute respiratory distress syndrome (ARDS).1 Patients with previous cardiovascular conditions seem to be at a higher risk for developing severe forms of COVID-19.1
Pulmonary arterial hypertension (PAH) is a rare disease with a poor long-term prognosis and a particularly high mortality risk during hospitalizations for noncardiovascular conditions.2
By 10 April 2020, 10 out of 350 PAH patients (100% female, 43.3 years [36.0-47.2]) followed up at our center were diagnosed with COVID-19.
All patients had a previous history of PAH (mean pulmonary artery pressure: 51.5 [44-66] mmHg; pulmonary vascular resistance 10.9 [8-18.4] Wood units). Median time from PAH diagnosis to COVID-19 was 3.9 [0.6–11.1] years.
A total of 7 patients (70%) required hospitalization (length of stay 10 [4-16] days), none in the intensive care unit. Five (50%) developed pneumonia, with ARDS features in 2 patients. Five patients (50%) needed oxygen therapy (3 of them were on domiciliary oxygen therapy). Clinical outcome was favorable in all patients (table 1).
Patients’ baseline characteristics before infection and COVID-19 clinical picture
| Pt | Sex | Etiology | Years | HTN | Smoker | MPAPmmHg | PVRWU | CIl/min/m2 | ESCrisk score | PAHtreatment | Picture | T°C | CXRpattern | CRPmg/dL | LDHu/L | ALCmL | DD ng/mL | LoSdays | O2 | COVID-19treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | HIV | 58 | N | N | 69 | 13.7 | 2.3 | Intermediate | PDiERAPCO2 | SOB | 40 | MLP | 1.9a | 265a | 1300 | 1324a | 16 | HFOb | LPV-RTVHCQ |
| 2 | F | FPVOD | 37 | N | N | 51 | 8.5 | 3.2 | Low | PDiERAO2 | SOB | 39 | MLP | 2.1a | 293a | 1200 | 455 | 14 | NSb | LPV-RTV |
| 3 | F | RCCB | 43 | N | N | 26 | 2.7 | 2.9 | Low | CCBs | Flu-like | 38 | Normal | 0.17 | 168 | 400a | 314 | 4 | None | LPV-RTVHCQ |
| 4 | F | IPAH | 47 | Y | Y | 28 | 2.5 | 3.1 | Low | PDiERA | Flu-like | 38.5 | Normal | 0.65 | NA | 1650 | NA | 0 | None | - |
| 5 | F | IPAH | 50 | N | N | 42 | 5.6 | 2.8 | Low | PDiERAPCO2 | SOB | 37.8 | MLP | 0.72 | 208 | 1520 | 266 | 6 | NSb | HCQ |
| 6 | F | RCCB | 35 | N | N | 29 | 1.9 | 3.3 | Low | CCBs | None | 36 | Normal | NA | NA | NA | NA | 0 | None | - |
| 7 | F | CHD | 76 | N | N | 44 | 5 | 3.24 | Intermediate | None | ARDS | 38 | MLP | 6.5a | NA | 1050a | 1300a | 28 | HFO | LPV-RTVHCQ |
| 8 | F | CTD | 64 | N | N | 26 | 2.7 | 3.2 | Low | ERA | ARDS | 38 | MLP | 4.2a | 228a | 430a | 520a | 20 | HFO | LPV-RTVHCQIL6-A |
| 9 | F | HIV | 53 | N | Y | 52 | 8 | 2.7 | Intermediate | ERA | Flu-like | 35.5 | Normal | 2.03a | 191 | 1500 | NA | 3 | None | LPV-RTVcHCQ |
| 10 | F | FPAH | 35 | N | N | 62 | 11.2 | 2.9 | Low | PDiERAPC | Flu-likeDiarrhea | 38.5 | Normal | 5.8a | 223a | 1300 | 396 | 0 | None | - |
ALC, absolute lymphocyte count; ARDS, acute respiratory distress syndrome; °C, degrees Celsius; CCBs, calcium-channel blockers; CHD, congenital heart disease; CI, cardiac index; CRP, C-reactive protein; CTD, connective tissue disease; CXR, chest X-ray; DD, D-dimer; ERA, endothelin receptor antagonist; ESC, European Society of Cardiology; F, female; FC, functional class (World Health Organization); FPAH, familial pulmonary arterial hypertension; FPVOD, familial pulmonary veno-occlusive disease; HCQ, hydroxychloroquine; HFO, high-flow oxygen; HIV, human immunodeficiency virus; HTN, hypertension; IL6-A, interleukin-6 antagonist; IPAH, idiopathic pulmonary arterial hypertension; LDH, lactate dehydrogenase; LoS, length of stay; LPV-RTV, lopinavir-ritonavir; MLP, multilobar pneumonia; MPAP, mean pulmonary artery pressure; N, no; NS, nasal spectacles; O2, domiciliary oxygen; PC, prostacyclin analogs or prostacyclin receptor agonists; PDi, phosphodiesterase type 5 inhibitors; pt, Patient; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RCCB, responders to calcium-channel blockers; SOB, shortness of breath; T, temperature; WU, Wood units; Y, yes.
We report the clinical characteristics and outcomes of PAH patients with COVID-19. The mortality risk from noncardiovascular processes for PAH patients is particularly high (9.4% for pneumonia, 21.4% for respiratory failure).2 Surprisingly, half of our patients only developed mild symptoms and, among those with established pneumonia, a favorable course was the general trend, without need for intensive care or deaths.
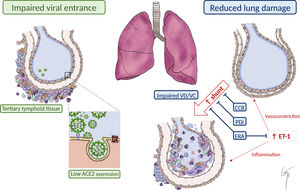
We hypothesize that the physiopathological features of PAH and the benefits attributable to specific treatment might lead to a protective effect through the following mechanisms (figure 1):
- 1.
Reduced viral entrance:
- –
Angiotensin-converting enzyme 2 (ACE2): In PAH patients’ blood and lungs, ACE2 expression is decreased.3 Indeed, recombinant ACE2 has been proposed as a novel therapy for PAH, to reverse vasoconstriction, proliferation, and inflammation.3 ACE2 is known to act as a receptor for SARS-CoV-2. Experimental studies with SARS-CoV have shown that in ACE2 knockout mice, only a very low quantity of infectious SARS-CoV virus could be recovered.4 Thus, low ACE2 levels in PAH patients could act as a protective factor at an initial infective phase, avoiding SARS-Cov-2 entrance.
- –
The role of chronic inflammation in PAH: Chronic pulmonary inflammation is a common finding in PAH patients.3,5 The immune cell types that infiltrate the lungs of PAH patients include lymphocytes, macrophages, neutrophils, dendritic cells, and mast cells. This different immune cellular landscape in PAH lungs suggests a shift toward the adaptive immune system. Therefore, the so-called tertiary lymphoid tissue is present in the vicinity of bronchioles and could limit viral infection and expansion.
- 2.
Attenuated lung damage: Changes in pulmonary circulation inherent to PAH pathophysiology or related to specific vasodilator treatment might reduce the damage inflicted to the lungs and the consequent severe hypoxemia described in COVID-19 patients.
- –
Changes in lung perfusion: An atypical form of ARDS has been described among COVID-19 patients. An unusual dissociation between lung mechanical properties (with nearly normal compliance) and severe hypoxemia has been reported,6 suggesting abnormal hyperperfusion of nonventilated areas as a consequence of impaired lung perfusion regulation and hypoxic vasoconstriction.6 The basal abnormal lung perfusion present in PAH patients could limit this abrupt perfusion imbalance toward nonventilated areas. Furthermore, chronic vasodilator treatment could prevent a severe hypoxic vasoconstriction response. In this regard, phosphodiesterase-5 inhibitors and even calcium-channel blockers have been proposed as a potential treatment for COVID-19, based on its vasodilator properties and a clinical trial with sildenafil is currently ongoing (NCT:04304313).1 Thus, pulmonary vasodilator therapy of our patients could have attenuated hypoxic vasoconstriction and have favored the ventilation/perfusion balance.
- –
Protective effect of endothelin receptor antagonists (ERAs) against ARDS: ARDS is caused by a severe inflammatory response, mediated by several proinflammatory agents and cytokines (tumoral necrosis factor, interleukins, or endothelin-1).5 Endothelin-1 has been shown to be involved in the pathogenesis of both ARDS and PAH.5 Previous reports suggest that ERAs might be useful in the treatment of ARDS, based on their beneficial effects in experimental preclinical studies.5 A total of 7 patients (70%) in our cohort received ERAs when diagnosed with COVID-19, thus suggesting the possibility of a beneficial effect of chronic endothelin-1 blockade.
Possible explanations for the benign course of COVID-19 in PAH patients. Impaired viral entrance to pulmonary cells due to the presence of “tertiary lymphoid tissue” and reduced ACE2 expression. Reduced lung damage due to impaired “vasotonic” properties and to PAH vasodilator treatment, minimizing intrapulmonary shunt. Reduced inflammatory response mediated by ET-1 due to the effect of ERA. ACE2, angiotensin-converting enzyme 2; CCB, calcium-channel blocker; ERA, endothelin receptor antagonist; ET-1, endothelin-1; PDi, phosphodiesterase-5 inhibitor; VD/VC, vasodilatation/vasoconstriction.
The clinical course of COVID-19 in our cohort of PAH patients was unexpectedly favorable. This finding could be explained by either the pathophysiological peculiarities of the disease or by a protective effect of PAH-specific treatment. PAH therapies may also have a protective effect in COVID-19, although that can only be addressed in placebo-controlled randomized controlled trials.
FundingJ. Nuche is recipient of a predoctoral grant (Jordi Soler Soler) through CIBERCV. P. Escribano Subías is recipient of a grant of the Spanish Ministry of Science and Universities for the study of molecular bases in pulmonary arterial hypertension (PI 18/01233). The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministry of Science and Innovation and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
Authors would like to thank Carlos Galán-Arriola (CNIC) for the design and drawing of the figure. We also would like to thank Javier Segovia-Cubero (Puerta de Hierro Hospital, Madrid), Francisco Pastor-Pérez (Virgen de la Arrixaca Hospital, Murcia) and Mercedes Alcalde (San Pedro Hospital, Logroño) for sharing the clinical records of patients admitted to their institutions.