Anomalous pulmonary venous drainage (APVD) is a congenital heart defect featuring the anomalous connection of either a single pulmonary vein (partial APVD) or all the pulmonary veins (total APVD), resulting in ectopic drainage outside the left atrium.1 The anomalously connected pulmonary vessels mostly drain into the venous system, generating a left-to-right shunt (in the absence of irreversible pulmonary hypertension). When severe, the ectopic drainage leads to enlargement of the right chambers.
Our group has achieved excellent interobserver and intraobserver reproducibility in the assessment of shunts using cardiac magnetic resonance (CMR) with 4D-flow.2 This imaging modality allows retrospective analysis of systemic and pulmonary flow at multiple planes in a single volumetric acquisition.3
Here, we show the results of 4D-flow CMR in 2 patients with partial APVD (PAPVD) before and after corrective surgery. All procedures were carried out with informed consent and were approved by the local hospital ethics committee. The first patient was an asymptomatic 26-year-old man, referred for CMR due to complete right bundle branch block detected by electrocardiography. Transthoracic echocardiography revealed right chamber enlargement, and CMR detected PAPVD in the superior and middle right pulmonary veins. The second patient was a 30-year-old woman, also asymptomatic, who was referred for CMR due to a heart murmur and right chamber enlargement detected by transthoracic echocardiography. CMR revealed PAPVD in the superior right pulmonary vein, which was associated with a superior sinus venosus atrial septal defect (SSV-ASD) and persistent left superior vena cava (PLSVC).
4D-flow CMR scans were performed with an Optima MR450w GEM 1.5T MRI scanner (GE-Medical-Systems, United States) with a 32-element antenna. Scans were acquired after infusion of 0.15 mmol/kg gadobutrol (Gadovist 1-mmol/mL, Bayer; The Netherlands) followed by saline solution. Velocity encoding was set at 150cm/s, to allow simultaneous assessment of venous and arterial flows; the mean temporal resolution was 29ms; and the voxel size was 2.0 × 2.0mm. The programmed 3D volume coverage spanned from the heart apex to the aortic arch. CMR scans were acquired during free breathing with retrospective electrocardiographic triggering. Sequence acquisition time was 7 to 9minutes.
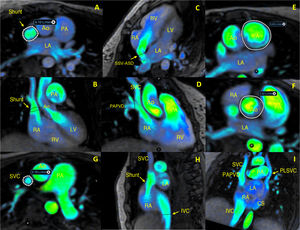
Presurgery 4D-flow CMR analysis of PAPVD allowed individualized assessment of pulmonary and systemic flows in any desired plane, as illustrated in figure 1 for patient 2, diagnosed with PAPVD, SSV-ASD, and PLSVC draining into the coronary sinus. The 4D-flow CMR analysis allowed direct qualitative and quantitative evaluation of the left-to-right shunting (SVC–PAPVD–SSV-ASD) (figure 1A,B), as well as separate assessment of the SSV-ASD (figure 1C) and the PAPVD (figure 1D). 4D-flow CMR also allowed quantification of systemic flow (figure 1E) and pulmonary flow (figure 1F), as well as the assessment of flow in the SVC (figure 1G), the inferior vena cava (IVC) (figure 1H), and the PLSVC (figure 1I).
4D-flow cardiac magnetic resonance analysis of a partial anomalous pulmonary venous drainage (PAPVD) before corrective surgery. Ao, aorta; CS, coronary sinus; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PA, pulmonary artery; PLSVC, persistent left superior vena cava; RA, right atrium; RV, right ventricle; SSV-ASD, superior sinus venosus atrial septal defect; SVC, superior vena cava.
Presurgery 4D-flow CMR thus not only provides an excellent anatomical and functional evaluation, but also permits quantification of the volume from the shunt and the systemic–pulmonary flow, as illustrated in figure 1:
- •
Pulmonary artery flow: 141mL/beat, 9.89 L/min; 87mL/beat, 6.10 L/min. Flow through the shunt: 34mL/beat, 2.39 L/min through the PAPVD; 11mL/beat, 0.78 L/min in the SVC; 44mL/beat, 3.07 L/min in the IVC; and 10mL/beat, 0.67 L/min in the PLSVC. The sum of flows in the shunt, IVC, and PLSVC correspond to the total flow in the pulmonary artery. The volume passing through the SSV-ASD was calculated indirectly (32mL/beat: shunt–SVC–PAPVD). Finally, determination of the systemic flow (71mL/beat, 5.02 L/min) allowed calculation of the pulmonary to systemic blood flow ratio (Qp:Qs)=2.
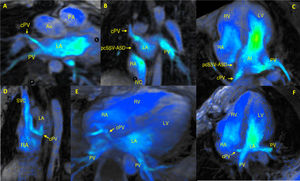
4D-flow CMR was equally vital for adequate evaluation after the corrective surgery, and the excellent results obtained are illustrated in figure 2. In patient 2, diagnosed with PAPVD and SSV-ASD (figure 2A-C and video 1 of the supplementary data), the postsurgery 4D-flow CMR results revealed optimal redirection of the pulmonary venous drainage (corrected pulmonary vein) to the interior of the left atrium. The analysis also confirmed the absence of interatrial flow at the level of the repaired ASD in the interatrial septum (figure 2B,C). In patient 1, diagnosed with PAPVD with no associated septal defect, postsurgery 4D-flow CMR revealed appropriate correction and redirection of drainage to the left ventricle through the interatrial septum (artificial ASD) (figure 2D-F and video 2 of the supplementary data). Neither patient showed evidence of velocity aliasing that would indicate stenosis of the corrected pulmonary vein, and both patients had a normalized postsurgery Qp:Qs ratio=1.
Postsurgery 4D-flow cardiac magnetic resonance analysis of corrected PAPVD. Ao, aorta; cPV, corrected pulmonary vein; IVC, inferior vena cava; LA, left atrium; LV, left ventricle; PA, pulmonary artery; pcSSV-ASD, patch-closed superior sinus venosus atrial septal defect; PV, pulmonary vein; RA, right atrium; RV, right ventricle.
4D-flow CMR, unlike standard 2D phase contrast CMR modalities, allows the assessment of arterial and venous flows in any of the 3 spatial dimensions through multiplane reconstruction of the acquired 3-dimensional volume. This ability is highly advantageous in decision-making on the treatment of simple or complex congenital heart defects both before and after surgical or percutaneous correction of the defect.4 The temporal resolution of CMR combined with the full coverage of the cardiac cycle (the fourth dimension in 4D-flow CMR) together permit excellent color visualization of flow patterns. Together with other advantages, such as the simultaneous assessment of anatomy and function and scan acquisition during free breathing, these features make 4D-flow CMR an essential tool for the routine assessment of these types of patients.
FUNDINGThis article did not receive specific funding.
AUTHORS’ CONTRIBUTIONSJ. Urmeneta Ulloa: diagnosis, planning, CMR postprocessing, and manuscript writing. J. Rivas Oyarzabal: surgical treatment of patients. J.A. Cabrera: manuscript supervision. A. Álvarez Vázquez: diagnosis, planning, CMR postprocessing. A. Forteza Gil: surgical treatment of patients. V. Martínez de Vega: diagnosis, planning, CMR postprocessing, and manuscript supervision.
CONFLICTS OF INTERESTThe authors declare no conflicts of interest.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.05.016