Although progressive dilation of the neoaortic root1 is less common than complications involving the neopulmonary root following arterial switch operation (ASO) to repair transposition of the great arteries in the neonatal period, it, together with neoaortic valve regurgitation, is a serious complication. Because the pulmonary branches are located in front of the ascending aorta after ASO (Lecompte maneuver, figure 1A-C), it is difficult to access the aortic root, unlike the situation when there is a normal spatial relationship between the aorta and the pulmonary artery. We present a small series of patients who underwent aortic root surgery for neoaortic valve regurgitation and/or dilation of the ascending aorta after ASO and describe arterial cannulation, aortic root access, and valve-sparing techniques.
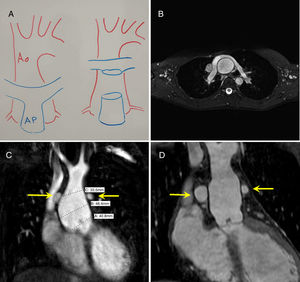
A, Diagram showing the pulmonary artery anterior to the aorta. B, Close-up preoperative axial section showing the Lecompte maneuver (pulmonary branches anterior to the aorta). C, Preoperative frontal view showing the pulmonary branches (arrows) and dilation of the aortic root. D, Postoperative frontal view showing the David procedure (patient #1).
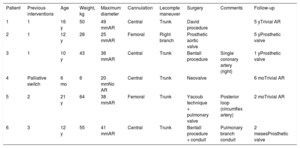
Six patients aged between 6 months and 21 years (median, 12 years) and weighing between 6 and 64kg (median, 43kg) with a history of ASO underwent surgery for neoaortic valve regurgitation (5 patients) and dilation of the ascending aorta (6 patients, all children) (table 1). Just 1 patient—the youngest in the series—had known risk factors for neoaortic root dilation.2,3 The patient was a 6-month-old infant who had undergone palliative ASO (previous aortic-pulmonary root size discrepancy and ventricular septal defect). The operation was the first reintervention for 4 patients, the second reintervention for 1 (previous neopulmonary valve replacement), and the third for another (2 previous neoaortic valve replacements). Chest computed tomography (figure 1) was performed to determine the spatial relationship between the great vessels (including the origin and path of the coronary arteries) and to check for adhesions to the sternum. A Doppler femoral ultrasound was also performed to assess the diameter and patency of the artery and vein.
Patients’ clinical characteristics
| Patient | Previous interventions | Age | Weight, kg | Maximum diameter | Cannulation | Lecompte maneuver | Surgery | Comments | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 16 y | 50 | 49 mmAR | Central | Trunk | David procedure | 5 yTrivial AR | |
| 2 | 1 | 12 y | 28 | 25 mmAR | Femoral | Right branch | Prosthetic aortic valve | 5 yProsthetic valve | |
| 3 | 1 | 10 y | 43 | 36 mmAR | Central | Trunk | Bentall procedure | Single coronary artery (right) | 1 yProsthetic valve |
| 4 | Palliative switch | 6 mo | 6 | 20 mmNo AR | Central | Trunk | Neovalve | 6 moTrivial AR | |
| 5 | 2 | 21 y | 64 | 38 mmAR | Femoral | Trunk | Yacoub technique + pulmonary valve | Posterior loop (circumflex artery) | 2 moTrivial AR |
| 6 | 3 | 12 y | 55 | 41 mmAR | Central | Trunk | Bentall procedure + conduit | Pulmonary branch conduit | 2 mesesProsthetic valve |
AR, neoaortic valve regurgitation.
Cannulation was femoral in 2 patients and central in 4. The pulmonary artery bifurcation (Lecompte maneuver) was mobilized in 5 patients using an inverse approach to the neonatal switch maneuver consisting of dissecting the bifurcation and moving both pulmonary arteries anterior to the ascending aorta. In the other patient, it was only necessary to separate and mobilize the right pulmonary artery to access the neoaortic root.
Neoaortic valve replacement was required in 3 patients as the valve was considered to be irreparable at the time of the intervention. One of the patients received a single prosthetic valve while the other 2 underwent valved conduit placement and reimplantation of the coronary arteries (just the right artery in 1 case) using the Bentall technique. Replacement of the ascending aorta with the valve-sparing David procedure (figure 1) and reimplantation of the coronary arteries with the Yacoub technique and Schäfers aortic annuloplasty were each performed in 1 patient. In the second case, the circumflex artery arose in the right coronary artery and followed a retroaortic path (type D, posterior loop) and was therefore very close to the area of the annuloplasty. Associated procedures included replacement of the neopulmonary valve with a valved conduit in 1 patient (third intervention) and replacement of both pulmonary arteries, both fragile, with a corrugated hilum-to-hilum conduit (fourth intervention) in another.
The case of the youngest patient in this series, aged 6 months and weighing 6kg, deserves special mention. The infant developed progressive neoaortic valve regurgitation due to neoaortic root dilation after an initially successful palliative ASO in the neonatal period to treat single-ventricle heart disease, aortic coarctation, subaortic stenosis, and transposition of the vessels. Although the valve appeared to be normal, its small size made it impossible to replace the ascending aorta and it was therefore decided to replace it with a heterologous pericardial “cylinder”.4 Trivial valve regurgitation was observed in 3 patients (David, Yacoub, and cylinder techniques) in successive follow-up visits; the prosthetic valves functioned normally in the other patients (table 1).
Progressive dilation of the neoaortic root (translocated in ASO) and associated valve regurgitation are serious complications.1–3 Neoaortic complications requiring surgery can be expected after ASO and they present later than neopulmonary complications. Arterial cannulation and access to the neoaortic root, however, are challenging due to the anatomy of the vessels following the Lecompte maneuver. With successive interventions, the pulmonary arteries can become fragile and may be found to require repair or replacement during surgery. Preoperative imaging tests (computed tomography and Doppler ultrasound of the femoral vessels) can also show the origin and path of the coronary arteries, providing essential information for remodeling or reimplantation techniques and situations in which one of the coronary arteries passes behind the root (patient #5 in our series). We favor valve-sparing techniques5,6 (David procedure [figure 1D] or Yacoub technique) in patients with normal-appearing valves and reserve replacement (prosthetic valves, Bentall procedure) for patients with dysplastic valves. Creative solutions are possible in cases of early-onset valve regurgitation after ASO.
We thank Dr Carlos Porras at Hospital Clínico in Málaga for his advice on valve-sparing aortic replacement techniques.