Sacubitril/valsartan, indicated for the treatment of chronic heart failure with reduced ejection fraction, was released on to the market in Spain in October 2016. It is the first available drug with a combined mechanism of angiotensin II receptor and neprilysin inhibition and with demonstrated superiority to enalapril for the composite outcome of cardiovascular mortality and hospitalization for heart failure.1 The main safety issue is hypotension.1
The study evaluating the safety and efficacy of sacubitril/valsartan had strict inclusion and exclusion criteria, so extrapolation of the results to clinical practice, especially the safety results, is not clear-cut. Both the clinical guidelines and the regulatory agencies recommend it for patients with a profile similar to those in the clinical trial: left ventricular ejection fraction ≤ 35%, high concentrations of natriuretic peptides, symptomatic despite optimal treatment, and with a good previous tolerance to angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin-II receptor blockers (ARB).2–4
A retrospective observational study was performed to analyze the use of sacubitril/valsartan in Catalonia in 2017 based on the Catalan Health Service (CatSalut) data file for pharmaceutical services. The study analyzed information on demographic variables (age and sex), dosage form used, concomitant heart failure treatments, and previous treatment with ACE-I and ARBs. It also evaluated the setting of the first prescription, adherence in terms of prescriptions dispensed compared with prescriptions issued, and the expenditure for CatSalut.
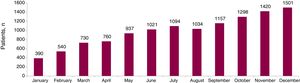
In 2017, sacubitril/valsartan was dispensed to 2179 patients (75.0% men) (Figure 1), at a cost of €2 739 365. The mean age was 69.8 years (men, 69.2; women, 71.8 years), with the following distribution: 20.1%, ≤ 60 years; 11.8%, 61 to 65 years; 35.6%, 66 to 75 years; and 32.4%, > 75 years. The dosage forms used are shown in Table 1.
Dosage Form of Sacubitril/valsartan Used
| Patients using only 1 dosage form | 1309/2179 (60.1%) |
| Dosage form of 24mg sacubitril and 26 mg valsartan (24/26 mg) | 845/1309 (64.6%) |
| Dosage form of 49mg sacubitril and 51 mg valsartan (49/51 mg) | 377/1309 (28.8%) |
| Dosage form of 97mg sacubitril and 103 mg valsartan (97/103 mg) | 87/1309 (6.6%) |
| Patients using more than 1 dosage form | 870/2179 (39.9%) |
| Dosage form switched from 24/26 to 49/51 mg | 385/870 (44.3%) |
| Dosage form switched from 49/51 to 97/103 mg | 247/870 (28,4%) |
| Dosage form switched from 24/26 to 49/51 and to 97/103 mg | 133/870 (15.3%) |
| Other changes | 105/870 (12.1%) |
Most patients (85.4%) with an active electronic prescription for sacubitril/valsartan in the month of December were treated concomitantly with a beta-blocker, and 60.7% were prescribed a beta blocker and aldosterone antagonist. The number of patients with a concomitant prescription for sacubitril/valsartan and an ACE-I (2) or an ARB (6) was very low. All patients except 1 had previously received an ACE-I or an ARB and 66.6% of prescriptions were issued by a specialist.
Regarding adherence, in 2017, 90% of prescriptions issued were dispensed. A total of 83.8% of patients had an adherence ≥ 80% and 58.5% had an adherence of 100%.
The number of patients treated with sacubitril/valsartan in Catalonia increased progressively throughout 2017 and has not yet stabilized. While the proportion of men and women treated is similar to that described in the clinical trial (75.0% vs 78.2% men), patients in clinical practice are older (mean, 69.8 vs 63.8 years).1
There was high use of the lowest dosage form, despite the summary of product characteristics stating that this is only indicated as a starting treatment in specific situations; a low percentage of patients were treated with the target dose. The tendency to use lower doses than in the clinical trials is common with other drugs acting on the renin-angiotensin system and has been described with sacubitril/valsartan.5 The practice is probably due to concern over hypotension in an older, more complex population. It is questionable whether the efficacy described at higher doses will be maintained under these conditions.
Prescription practices were compatible with the recommendations in terms of previous and concomitant treatments, although investigation is required to explain why 40% of patients were not receiving triple therapy with sacubitril/valsartan, a beta-blocker, and an aldosterone antagonist. The high adherence to the recommendation to stop ACE-I and ARBs to avoid safety issues could be related to the introduction of alerts on the CatSalut electronic prescribing system.
The main limitations of this study are the absence of information on prescriptions issued in private health care or paper prescriptions, which could have led to underestimation of the percentage of treatments started by specialists; not having analyzed the dose of concomitant treatments, which means we do not know if they were optimized; the use of an indirect measure of adherence that does not tell us whether the patients really took the medication; and, above all, the lack of clinical data (left ventricular ejection fraction or New York Heart Association functional class).
In conclusion, despite these limitations, the data on use in Catalonia suggest that the recommendations on sacubitril-valsartan prescribing are being followed. The patients treated are older than those in the PARADIGM-HF trial1 and are generally prescribed lower than recommended doses, without evidence to support this. Considering that the number of patients treated with sacubitril/valsartan is expected to continue to increase over the coming years, assessment of health outcomes is particularly relevant.