Extracorporeal membrane oxygenation (ECMO) systems are circulatory support devices that provide effective hemodynamic support in patients with cardiogenic shock with left ventricular dysfunction.1,2 There are, however, other less well known options for their use, such as right ventricular failure with cardiogenic shock,3 hemodynamic support during high-risk percutaneous coronary intervention (PCI),4 and life-threatening electrical storm.5
Between October 2013 and December 2014, the CARDIOHELPTM system (MAQUET Cardiopulmonary AG; Germany) was used to perform 10 venoarterial and 3 venovenous ECMO procedures in patients at the University Hospital of Salamanca (Spain). We describe the baseline characteristics, indications, implantation approach, ECMO duration, management, and progress of 5 ECMO patients whose indications were other than cardiogenic shock (Table).
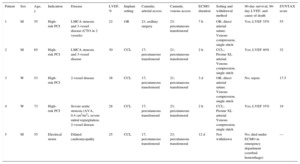
Baseline Characteristics, Indication, Implantation Route, Management, and Outcome of Patients Provided with Ventricular Support via Venoarterial Extracorporeal Membrane Oxygenation
| Patient | Sex | Age, y | Indication | Disease | LVEF, % | Implant setting | Cannula; arterial access | Cannula; venous access | ECMO duration | Setting and withdrawal method | 90-day survival, 90-day LVEF, and cause of death | SYNTAX score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 55 | High-risk PCI | LMCA stenosis and 3-vessel disease (CTO in 2 vessels) | 22 | OR | 21; axillary surgery | 23; percutaneous transfemoral | 7 h | OR; direct arterial suture. Venous compression, single stitch | Yes; LVEF 35% | 55 |
| 2 | M | 65 | High-risk PCI | LMCA stenosis and 3-vessel disease | 30 | CCL | 17; percutaneous transfemoral | 21; percutaneous transfemoral | 2 h | CCL; Prostar XL arterial. Venous compression, single stitch | Yes; LVEF 40% | 32 |
| 3 | W | 53 | High-risk PCI | 2-vessel disease | 38 | CCL | 17; percutaneous transfemoral | 21; percutaneous transfemoral | 3 d | OR; direct arterial suture. Venous compression, single stitch | No; sepsis | 17.5 |
| 4 | W | 73 | High-risk PCI | Severe aortic stenosis (AVA, 0.4 cm2/m2), severe mitral regurgitation, 2-vessel disease | 28 | CCL | 17; percutaneous transfemoral | 23; percutaneous transfemoral | 2 h | CCL; Prostar XL arterial. Venous compression, single stitch | Yes; LVEF 35% | 19 |
| 5 | M | 55 | Electrical storm | Dilated cardiomyopathy | 25 | CCL | 17; percutaneous transfemoral | 23; percutaneous transfemoral | 12 d | Not withdrawn | No; died under ECMO in emergency department (cerebral hemorrhage) | — |
AVA, aortic valve area; CCL, cardiac catheterization laboratory; CTO, chronic total occlusion; ECMO, extracorporeal membrane oxygenation; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; M, man; OR, operating room; PCI, percutaneous coronary intervention; W, woman.
Extracorporeal membrane oxygenation was administered for hemodynamic support during high-risk PCI in 4 patients and during a life-threatening electrical storm in 1 patient. All patients were discussed by a heart team comprising cardiologists and cardiac surgeons. Heart surgery was initially ruled out in all patients. Three patients were scheduled for intervention; however, because of patient 3's clinical status, the procedural approach was agreed in the catheterization laboratory. Interventional cardiologists and cardiac surgeons jointly performed cannulation and a perfusionist operated the CARDIOHELPTM system. During all procedures, ECMO support was maintained at a flow of 2.5/L min to 3.0 L/min with an activated clotting time between 180 seconds and 250 seconds. With the exception of patient 2, all patients required invasive mechanical ventilation. Intra-aortic balloon counterpulsation was used in 2 patients (patients 3 and 5) to detect signs and symptoms of heart failure without shock.
Extracorporeal membrane oxygenation was administered in patient 1 to provide support during a PCI procedure, which was considered high risk based on severe left ventricular dysfunction, 3-vessel disease with total occlusion of 2 vessels, liver disease, severe sleep apnea-hypopnea syndrome, and severe peripheral vascular disease. Given the latter condition, a hybrid method was used to administer ECMO. In the operating room, surgical access was obtained through the right axillary artery and percutaneous access through the right femoral vein. The patient was transferred to the cardiac catheterization laboratory and ECMO was removed without complications in the operating room after conclusion of the PCI procedure. The patient's progress was favorable.
In patient 2, ECMO was also used to provide support during a PCI procedure, which was considered high risk based on Killip class III acute myocardial infarction, left main coronary artery and 3-vessel disease, severe left ventricular dysfunction, and kidney failure. Extracorporeal membrane oxygenation was administered through peripheral access in the cardiac catheterization laboratory. Similar to the procedure used in patient 4, a Prostar XL (Perclose, Abbott Vascular Devices) device was used to achieve hemostasis prior to ECMO arterial cannula insertion. The patient's progress was favorable.
Patient 3 received urgent ECMO. This patient had acute anterior myocardial infarction treated with primary angioplasty with a stent in the proximal left anterior descending artery. During a second revascularization procedure of the proximal right coronary artery, the patient showed severe iatrogenic dissection of the proximal left anterior descending artery, TIMI (Thrombolysis In Myocardial Infarction) 0 flow, hemodynamic instability, and acute pulmonary edema, requiring endotracheal intubation and intraaortic balloon pump implantation. Given the patient's hemodynamic instability, ECMO was administered percutaneously in the cardiac catheterization laboratory and the right coronary artery was revascularized without complications. Extracorporeal membrane oxygenation was surgically removed on the third day due to improvements in the patient's hemodynamic status. One week later, the patient died from complications of hospital-acquired pneumonia.
Patient 4 had aortic stenosis, severe mitral regurgitation, 2-vessel coronary disease, severe left ventricular dysfunction, and heart failure. Aortic valvuloplasty supported by ECMO was performed as a bridge to cardiac surgery. Extracorporeal membrane oxygenation was administered through peripheral access in the cardiac catheterization laboratory. One month later, the patient underwent further intervention. Based on improved left ventricular dysfunction and the absence of heart failure, aortic and mitral valve replacement and double coronary artery bypass grafting were performed with good outcome.
Extracorporeal membrane oxygenation was administered in patient 5 following an electrical storm with hemodynamic instability that did not respond to conventional management. Extracorporeal membrane oxygenation was administered percutaneously in the cardiac catheterization laboratory and the superficial femoral artery was cannulated to perfuse the distal limb and prevent ischemic complications. Following the method described in Revista Española de Cardiología,6 the patient was transferred 224 km under ECMO support to a heart transplant center, where he remained in the emergency department for 10 days until dying from an intracranial hemorrhage.
With the exception of cardiogenic shock,1,2 experience with ECMO as hemodynamic support in adults has not been published in Spain. We present the potential usefulness of percutaneous implantation of ECMO support in high-risk PCI, and even in immediate life-threatening electrical storm, although studies with more patients are needed. Extracorporeal membrane oxygenation provides hemodynamic support and decreases the risk associated with percutaneous procedures in patients with multiple comorbidities. It has even allowed us to move a patient with electrical storm and hemodynamic instability over a considerable distance to a heart transplant center. In our opinion, ECMO could provide an alternative means of hemodynamic support beyond the setting of cardiogenic shock, and in cardiology services where PCI or highly complex electrophysiological studies are performed.
FUNDINGThis study was partly funded by the European Regional Development Fund/Instituto de Salud Carlos III, and the Red de Investigación Cardiovascular (RD12/0042).