Hypertrophic cardiomyopathy (HCM) is a genetic disease caused by mutations in at least 11 genes that primarily code for cardiac sarcomere proteins.1 Historically, the management of this disease has involved systematic evaluation of the individual's first-degree relatives over many years. Since the first report of a mutation associated with HCM in 1990, genetic analysis has progressed from research to clinical practice. Currently, knowledge of the causative mutation in an individual with HCM does not usually imply changes to the therapeutic approach. However, detection of the genetic defect can have important implications, because patients and their families can be offered effective genetic counselling.2 Furthermore, early identification of the mutation in relatives allows closer monitoring and early detection of complications. Finally, relatives without the mutation can be excluded, thereby avoiding unnecessary follow-up.1,2 The systematic use of genetic testing for HMC has been shown to be cost-effective in theoretical models applied in Anglo-Saxon countries,3 but no data are available from Spain.
The aim of the present study was to evaluate the clinical and economic impact of genetic analysis of the relatives of HCM patients in Spain. To do this, we performed an observational study of families with HCM who underwent genetic testing from 2008 to 2011 in 2 inherited cardiovascular disease units.
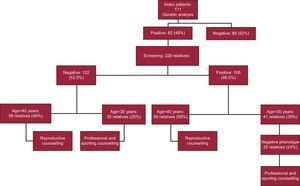
We evaluated the clinical utility of genetic testing (reproductive, professional, and sports counselling) and the direct economic savings derived from the cessation of follow-up of relatives with a negative test result. During the study period, genetic testing was performed in 171 index patients with HCM (118 [68%] men; mean age, 52 [17] years). In all patients, the exons and intronic flanking regions of the 5 genes most frequently involved in HCM (MYH7, MYBPC3, TPM1, TNNI3, and TNNT2) were analyzed using Sanger sequencing. A further 4 genes (ACTC, MYL2, MYL3, and TNNC1) were analyzed in 43 index patients (25%), and 2 further genes (PRKAG2 and LAMP2) were analyzed in 4 patients. The causative mutation was identified in 82 index patients (48%). Patients who had variants of uncertain significance were excluded from the study.
A clinical evaluation (with electrocardiograms and echocardiograms) plus cascade genetic screening was then undertaken in relatives who wished to be included in the study. In total, 228 family members were evaluated (2.8 relatives/index patient; mean age, 39 [19] years). Of these, 106 relatives (46.5%) were carriers of the same mutation as the index patient (positive genotype). In contrast, 122 relatives (53.5%) did not have the mutation (negative genotype) and, after receiving genetic counselling, were given definitive clearance (Figure).
Genetic counselling includes reproductive, professional, and sports advice. The assessment team should include experienced professionals. The risks and benefits of testing, as well as its clinical, psychological, and social implications, should be clearly explained to patients and their relatives. Reproductive counselling should ideally be completed before pregnancy. Counselling should address issues such as the inheritance pattern, penetrance, expressivity, and familial history. All participants found to be carriers of a causative mutation must be informed of alternative reproductive methods.
To evaluate the study participants who would benefit from reproductive advice, the cut-off for reproductive age was set at 40 years. Thus, 42% of the participants with mutations (78 patients) were younger than 40 years and therefore received reproductive counselling. Relatives who were noncarriers also benefited from reproductive counselling. In the present study, almost half (48%) of the relatives with a negative genotype (59 relatives) were younger than 40 years and were therefore informed that their descendants would not have the disease (Figure).
The European Society of Cardiology advises against participation in competitive sports activity in persons with a positive genotype and negative phenotype,4 but provides no recommendations on professional activities. However, given the high probability that genetic carriers will develop the phenotype at some point in their lives, it seems reasonable to advise against professions that HCM patients would be unable to carry out (police, fire-fighter, pilot, etc.), as well as against participation in competitive sports involving intense physical activity.
In the study population, 25 relatives with a positive genotype and a negative phenotype (24%) and 30 relatives with a negative genotype (25%) were younger than 30 years old and were therefore advised about their profession and sporting practices (Figure).
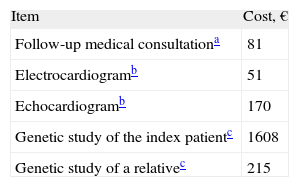
Finally, we determined the economic costs of genetic testing, such as the difference between the cost of genetic tests vs the savings in consultations, electrocardiograms, and echocardiograms among genotype-negative relatives who discontinue the periodic monitoring recommended by clinical practice guidelines.1,2 The costs of the genetic analysis and other tests were calculated from the mean rate charged by 2 companies and by the official costs in Spanish autonomous communities (Table).5,6
After detailed clinical investigation to identify families who could potentially have multiple mutations, 122 relatives with the negative genotype were given definitive clearance.
The total expenditure on genetic screening was €323 988. As shown in the Table, the savings derived from the cessation of monitoring of genotype-negative relatives amounted to €300 913 (€3167 [1906] per relative participating in the study).5,6 The estimated direct cost of genetic analysis (cost of genetic analysis minus direct health care savings) was €23 075. The cost of each relative undergoing genetic analysis was therefore €135.
The present study demonstrates both the clinical and economic viability of genetic testing for HCM in Spain. This study did not consider the potential savings in other types of test (cardiac magnetic resonance imaging performed in unclear cases) or other indirect benefits (days off work, relief of anxiety) that are also derived from discontinuation of follow-up. Equally, this study did not include the costs that would be saved by performing genetic analysis within the hospital instead of outsourcing the work to private companies. Finally, this study was based on the technology for genetic investigations available from 2008 to 2011. New ultra-sequencing techniques, allowing a greater number of genes to be analyzed at a lower cost, will probably enhance the favorable cost/utility balance of genetic testing.
FUNDINGThis study was partially financed by the Instituto de Salud Carlos III (PI11/0699, RD12/0042/0066 and RD12/0042/0069).