Keywords
TRICUSPID VALVE DISEASE
Tricuspid valve (TV) is often referred to as theforgotten valve and is considered deserving ofmore respect. However, recent literature is repletewith emphasis on TV disease and its long term implications.1-4 A variety of surgical approacheshave been introduced. Intermediate and longterm outcome of some of these surgical optionsis promising.5 The present report will attempt tosummarize the current understanding of the TVdisorders in terms of diagnosis, prognosis andtreatment.
Embryology
The septation of atria and ventricles in thefetal circulation is followed by formation ofendocardial cushions at the crux of the heart. Theatrioventricular (AV) valves develop subsequently.The architecture of the two AV valves is intimatelytied to the corresponding ventricles. This relationshipdemonstrates that the mitral valve is connected to theanatomic left ventricle and the TV to the anatomicright ventricle. This relationship is emphasized inthe congenitally corrected transposition of greatarteries with functionally intact circulation, suchthat the anatomic right ventricle becomes thesystemic ventricle and anatomic left ventricle thepulmonary ventricle. The corresponding AV valvesare transposed along with the ventricles. Thus, theTV becomes a left sided valve between the left atriumand anatomic right ventricle, which is the systemicventricle connected to the aorta. Similarly the mitralvalve is transposed with anatomic left ventricle,which is the pulmonary ventricle connected to thepulmonary artery and low resistance pulmonarycirculation. Congenitally corrected transposition inabsence of other malfunctions is compatible withlife into the sixth and seventh decades. This speaksto the adaptation of the anatomic right ventricleand TV to high pressure, high resistance systemiccirculation.
Since the formation of AV cushions at the cruxof the heart are central to distinctive anatomy of the two atrioventricular valves, the congenitalabsence of AV cushions, partial or complete, resultsin striking abnormalities of the two AV valves.The attachment of the septal leaflet of the TV isnormally more apical than the mitral valve, and asmall defect is capable of providing shunting ofblood from the left ventricle to the right atrium.This defect, when isolated, is anatomically smalland is known as Gerbode defect. Another anatomicconsideration is that the septal leaflet of the TV isattached to membranous ventricular septum. Thus,perimembranous ventricular septal defects distortthis portion of the TV, which can grow over thedefect, resulting in spontaneous closure of smallmembranous VSD in childhood.
Valve Anatomy
The TV is most caudally located and has the largestorifice of the 4 intracardiac valves. It functions asa unidirectional valve permitting systemic venousblood flow from right atrium and hence from the 2vena cava to advance to the right ventricle duringdiastole and prevents backflow or regurgitationduring systole. The TV apparatus is composed ofthe annulus, the leaflets, the chordae and papillarymuscles. Its coordinated function is also influencedby the geometric alterations of the right ventricleand the right atrium.
The annulus: The tricuspid annulus is oval in shape, but assumes a more circular shape ondilatation. It has been shown to have a more complexnonplanar shape with postero-septal commissurethe highest point. The shape, besides becoming morecircular, flattens out and becomes more planar inpresence of severe "functional" regurgitation. Theannular diameter, circumference and area are alllarger than the mitral valve by about 20 percent.Although major tricuspid annular diameter valuesof 30-35 mm are described for normal adults (BSA 1.5-1.7 m2), the orifice size is influenced by overallbody size as reflected in body surface area. Thus,while a measured diameter of 40 mm in an averagesize normal adult represents dilated annulus, thismay be normal for a person with BSA in excessof 2.0 m2. Thus, size of an individual patient mustbe considered in assigning the given measure asnormal or abnormal. The average normal annulardiameter is 21 (2) mm/m2. A hemodynamicconsequence of a larger tricuspid annulus orificeis lower velocities and lower pressure drops duringdiastolic inflow than in the normal mitral valve.The annulus exhibits a dynamic behavior similarto the mitral annulus, with expansion of the orificein diastole and reduction in systole.6 The maximumto minimum area reduction is nearly 30%. Thisdynamic behavior promotes forward flow while maintaining low right atrial and thus systemicvenous pressures.
The TV has 3 leaflets; anterior, septal and posterior,the anterior being the largest and septal being thesmallest. The septal leaflet attachment is fromposterior ventricular wall across the interventricularseptum, its insertion being more apical relative tothe anterior leaflet. The anterior leaflet is attachedto the right AV junction. The posterior leaflet hasmural attachment.
The tendinous chords are attached to theventricular surface of the leaflets or the free edgesof the leaflets to the papillary muscle supporting theleaflet. There may be accessory chords that attachfrom the septal leaflet to the moderator band or theright ventricular free wall.
There are 3 sets of papillary muscles, each setbeing composed of up to 3 muscles. The chordaearising from each set are inserted into 2 adjacentleaflets. Thus, the anterior set of chordae insertinto half of the anterior and half of the posteriorleaflets, the medial set provides chordae to anteriorand septal leaflets. The third, posterior, set is morerudimentary and is attached to the diaphragmaticwall of the right ventricle.7
Normal Tricuspid Valve Function
The diastolic opening of the valve along withcorresponding expansion of the annulus provides atricuspid orifice area of 7-9 cm2. This large orificeprovides unimpeded flow both at rest and withphysical activity without elevations in central venouspressures. The systolic narrowing of the orificeprovides an effective seal for valve closure; however,a measure of tricuspid regurgitation (TR) detectedby Doppler echocardiography is observed in 80%-90% of normal subjects. The majority of patientswith physiologic TR are in the mild category, but asmall number of otherwise healthy individuals mayhave moderate regurgitation. A failure to appreciatethis may result in identifying as abnormal what is anormal variant.
Tricuspid Valve Dysfunction
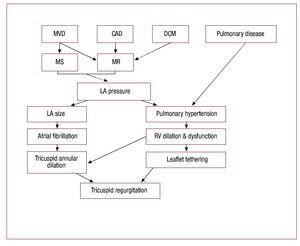
The TV disease is generally classified as primary orintrinsic valve pathology or secondary or functionalvalve dysfunction.8,9 The primary valve diseaseresults from structural abnormality of the valveapparatus. The secondary or functional TV diseaseresults from factors that generally lead to tricuspidannular dilatation, commonly from left heartdisease and resulting right ventricular hypertension,dilatation and dysfunction (Figure 1).10
Figure 1. Pathogenesis of functionaltricuspid regurgitation. CAD, coronary artery disease; DCM,dilated cardiomyopathy; LA, left atrial; MS,mitral stenosis; MR, mitral regurgitation;MVD, mitral valve disease. Modified fromShiran et al.10
A classification of TV disease according to etiologyis given in Table 1.
Clinical Presentation
The abnormal valve function may be in form of: a) pure or predominant tricuspid stenosis; b) pureor predominant TR; or c) mixed.
Generally the symptoms of left heart diseasepredominate in those with secondary TV disease.The symptoms specific to advanced TV diseaseare related to: a) decreased cardiac output, forexample, fatigue; b) right atrial hypertension,for example, liver congestion resulting in rightupper quadrant discomfort, or gut congestionwith symptoms of dyspepsia, indigestion, or fluidretention with leg edema and ascites. It may beemphasized that significant TV disease may not beassociated with any symptoms until a late stage ofthe disease involving progressive right ventriculardysfunction. Symptoms caused by underlyingetiology such as flushing, diarrhea, abdominalpain, etc. associated with carcinoid heart diseasepoint to the etiology.
Physical findings include signs related to TVdisease and those secondary to chronic venouscongestion, that is, leg edema and ascites. Tricuspidstenosis results in characteristic changes in the jugularvenous pulse in form of a slow "V" to "Y" descentand prominent "A" waves. The liver is enlarged witha firm edge, and pulsatile in presystole. Auscultationreveals a low-to-medium-pitched diastolic rumblewith inspiratory accentuation. This is usuallylocalized to the lower sternal border.11
TR results in the jugular venous pulse exhibitinga prominent "C-V" wave or systolic wave. Thereis often a parasternal lift from right ventricularenlargement. The liver shows systolic pulsations, isenlarged and often tender. The cardiac auscultationreveals a soft early or holosystolic murmur whichis augmented with inspiratory effort (Carvallosign). A systolic honk may be present with TVprolapse.12 Substantial TR may exist without theclassic ausculatory findings. Thus, neither presencenor quantitation of TR can be reliably judged byauscultation. The pulsatile liver is a sign of severeregurgitation.
Laboratory Diagnosis
Electrocardiogram: There are no specificmarkers of TV disease, although the followingclues may be present: a) right ventricular (RV)hypertrophy and "strain" with right QRS axis;and b) right atrial enlargement with prominentP waves. Specific ECG signs of primary etiologymay be noted, such as left axis deviation andcomplete right bundle branch block in AV canaldefect associated with cleft valve, and Ebstein'sanomaly may exhibit wide QRS.
Chest radiograph: cardiomegaly associated withprominent right-heart borders may be noted. Thereare no specific findings to suggest a diagnosis of TVdisease.
Echocardiography: 2-dimensional echocardiogramcombined with spectral and color flow Dopplerevaluation provides the most accurate laboratorytest in detection and quantitation of TV disease.
In addition, the TV morphology provides clues ofunderlying etiology and pathophysiology of valvedysfunction.13
Tricuspid valve morphology: Ebstein's anomalyis characterized by apical displacement of theseptal tricuspid leaflet into the right ventricle bymore than 8 mm/m2 from the insertion point ofthe anterior mitral leaflet from the crux. The rightatrium is enlarged, composed of anatomic rightatrium proper and atrialized proximal inflow rightventricle. The residual right ventricle is reduced insize.
AV cushion defect with associated cleft valveabnormality is best seen in apical 4 chamber view.The mitral and TVs are seen as a common valvestraddling the defect. The cleft may be visualizedwith confirmation by color flow image showingthe regurgitation jet going across the valveabnormality.
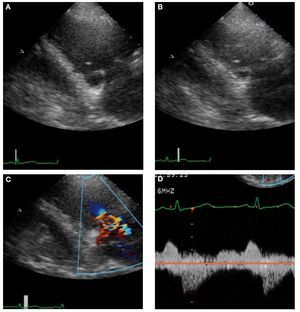
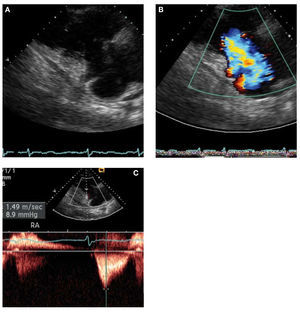
Carcinoid heart disease is characterized bythickened immobile valve leaflets held in half openposition, resulting in appearance of stenosis as wellas free flowing regurgitation with color flow Doppler(Figure 2).14
Figure 2. Echocardiographic images in 48-year-old man with carcinoid heart disease. A: end-diastolic frame showing the tricuspid valve (TV) in the openposition. B: the TV in the end-systolic frame is in a partially open position. C: this characteristic valve restriction results in severe tricuspid regurgitation. D: thecontinuous-wave Doppler shows several characteristic features. The systolic velocity of early peak and rapid deceleration indicate high right atrial pressure.The diastolic velocity with slow early deceleration and a prominent presystolic flow is consistent with some degree of tricuspid stenosis.
Rheumatic TV disease is nearly always associatedwith rheumatic mitral and/or aortic valve disease.The valve leaflets are thickened and exhibit somedoming in diastole.
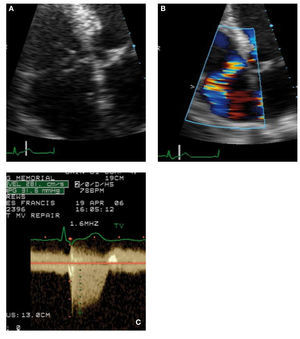
TV prolapse is seen in nearly 30% of patients withmitral valve prolapse. The characteristic appearanceincludes dilated annulus, billowing prolapse,less commonly chordae rupture with flail leaflet(Figure 3).14 Apart from the general syndrome ofdegenerative valve disease, TV prolapse has beendescribed in congenital heart disease associated withsystemic right ventricles.
Figure 3. A 56-year-old man with mitral valve prolapse exhibitingspontaneous rupture of the tricuspid valve. A: the flair septal leafletof the tricuspid valve (TV) is shown in systole. B: the resulting eccentrictricuspid regurgitation (TR) jet directed laterally because of flail TV. C:the continuous-wave Doppler recording of the TR jet with a peak systolicgradient between right ventricle and right atrial of 32 mm Hg. The inferiorvena cava was measured at 2.5 cm and showed no discernible collapse,indicating estimated right atrial pressure of 20 mm Hg. Thus, the rightventricular systolic pressure is estimated as 52 mm Hg.
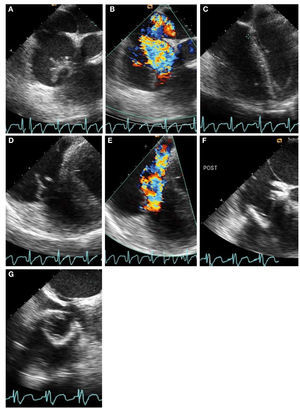
Infective endocarditis is generally apparentwith demonstration of mobile vegetation withtransthoracic echocardiography. In some cases,the transesophageal approach may be used forconfirmation. Differentiation of a vegetation from atumor requires clinical correlation (Figure 4).14
Figure 4. Tricuspid valve endocarditis in a 35-year-old man with positive blood cultures growingStaphylococcus aureus. Intraoperative transesophageal echocardiography shows salient featuresbefore and after tricuspid valve (TV) replacement. A: large irregular vegetation prolapsing into the rightatrium is systole. B: severe tricuspid regurgitation (TR) associated with TV endocarditis. C: tricuspidannulus measures 4.5 cm and is markedly dilated as a consequence of right ventricular and rightatrial dilatation. D: the anterior TV leaflet is flail. E: the TR jet is deflected into the coronary sinusas a result of flail TV. F and G: a bioprosthesis is placed in the TV position shown in short-axis and4-chamber views.
Valvulopathy associated with Phen-Fen andmethysergide consists of thickened, fibrotic, lessmobile tricuspid leaflets. These appearances arenon-specific and require historical confirmation ofdrug use.
Pacemaker lead related trauma exhibits leafletentrapment by a pacemaker lead. The color flow jetof TR may be localized at the pacemaker contact sitealong the tricuspid leaflet. Less commonly, leafletperforation may be noted.
Secondary or functional TR is characterized byannular dilatation, generally the annular diametergreater than 40 mm, and tethering of leaflets withtenting distance in excess of 8 mm. In extremecases, the leaflets fail to coapt with wide open regurgitation. Severe right ventricular hypertensionis associated with shift of the interventricular septumtoward the left ventricle, resulting in asymmetrictethering. In addition, characteristic appearancesof right ventricular infarction, arrhythmogenicright ventricular dysplasia, or myocarditis andcardiomyopathy may be observed.
Detection and Quantitation of Tricuspid ValveDisease
Color flow Doppler and spectral Doppler aresensitive for detection of valve regurgitation andgenerally accurate for semiquantitative assessmentof tricuspid stenosis and regurgitation.15 Tricuspidstenosis is detected with color flow imaging bydemonstrating a central core of high velocity jet. Thecontinuous wave Doppler permits measurements ofmean and end-diastolic gradients. The normal meangradient is less than 3 mm Hg and the end diastolicgradient nearly zero. Severe stenosis is associatedwith mean gradient of 5 mm Hg and pressure halftime measured in end inspiratory beat is greater than190 ms. It has been proposed, but not well validated,that TV area may be determined by 190 divided bypressure half time.
TR using color flow imaging is readily recognizedfrom parasternal tricuspid inflow view, short axisview, and apical or subcostal four chamber crosssections. Regurgitant jet area correlates roughly withseverity of regurgitation, being less than 5 cm2 inmild, 6-10 cm2 in moderate and greater than 10 cm2 in severe cases. In clinical practice, a visual estimaterather than actual planimetry is utilized. A moreaccurate estimate may be obtained by utilizing flowacceleration and PISA (Proximal Isovelocity SurfaceArea) measurements from which regurgitant orificearea may be calculated. The measured PISA radiusis by itself a good guide to severity of regurgitation.The technique is important. The color flow baselineshould be shifted in direction of regurgitation toget aliased velocity of approximately 30 cm/sec.The radius of hemispherical PISA of greater than9 mm indicates severe, 5-9 mm moderate, and lessthan 5 mm mild regurgitation. The spectral Dopplerimage of TR represents pressure gradient betweenright ventricle and right atrium through systole. Theshape of TR velocity profile using continuous waveDoppler provides a clue to this relationship. Theregurgitation profile is generally parabolic exceptin severe cases, where high right atrial "C-V" wavesresult in rapid equalization with right ventricularpressure giving a profile with rapid deceleration,also described as 'V' wave cut off sign (Figure 5).14
Figure 5. Severe secondary tricuspid regurgitation caused by extremetethering of the tricuspid valve without intrinsic leaflet pathology. A: theright ventricular inflow view from parasternal transducer location shows amarkedly tethered valve in late systole. B: the color Doppler image showsflow accelerating and severe tricuspid regurgitation jet without turbulence.C: continuous-wave Doppler shows early peaking systolic profile associatedwith high right atrial pressure, which was estimated to be 25 mm Hg. Thepeak tricuspid regurgitation velocity is measured at 9 mm Hg and thusindicates the right ventricular systolic pressure to be 34 mm Hg (9 + 25).
Additional indirect clues of regurgitation severityare density of continuous wave Doppler profile,size of right ventricle and atrium, paradoxical interventricular septal motion, and systolic bulge ofinteratrial septum toward left atrium. The hepaticvein flow may exhibit systolic reversal of flow insevere cases.
A calculation of right ventricular systolic pressure(ie, pulmonary artery systolic pressure in absence of outflow obstruction) using peak TR velocity isextremely useful in clinical practice. The formulaused is: Right Ventricular Systolic Pressure = 4 x TR velocity + right atrial pressure.16 The latter may be assumed to be 7-10 mm, or more accuratelydetermined from size of inferior vena cava and its collapse with sniff test. It is important to emphasizethat height of TR velocity is not indicative ofseverity of regurgitation, but rather the degree ofright ventricular systolic pressure or pulmonaryhypertension.
Transesophageal echocardiography (TEE):transthoracic echocardiography is often of diagnostic quality because the TV and the rightventricle are closer to the anterior chest wall andseveral parasternal, apical, and subcostal viewsare used to image these structures. However,TEE is indicated for better anatomic definitionsof the valve lesions or precise measurement of thetricuspid annulus. The assessment of severity of tricuspid stenosis or TR is generally more accuratewith transthoracic echocardiography. This isespecially true in the intraoperative setting, whereseverity of TR may be underestimated as a resultof lowered pulmonary vascular resistance from theanesthetic agents. It is therefore erroneous to usethe severity of TR in the operating room to decideif a surgical procedure is to be performed on theTV. In the intraoperative setting, TEE is especially used for measuring the tricuspid annulus diameter.This is done in the midesophageal four-chamberview and a plane perpendicular (90 degrees) to it.
Cardiac catheterization and selective angiography:Prior to the advent of diagnostic echocardiography,cardiac catheterization was used to confirm thepresence and severity of tricuspid stenosis. It wasrecognized that simultaneous recordings of right atrial and right ventricular diastolic pressures wasneeded for accurate assessment because the pressuregradients are small and there is considerablerespiratory variation in the pressure wave forms.The diagnosis of TR posed a greater challenge, asselective angiography into the right ventricle wouldoften distort the TV. The pressure wave form in theright atrium shows the characteristic prominentsystolic V wave with rapid descent only in the mostsevere cases. Diagnostic cardiac catheterizationshould rarely, if ever, be undertaken for the diagnosisor quantitation of TV disease alone.
Treatment
The treatment of TV disease must entertain twoimportant questions, namely when to treat and howto treat (Table 2).
When to Treat?
The decision to treat TV disease is based largely onhemodynamic and functional consequences of thediseases as well as coexistence of other associatedvalvular or congenital lesions. As an isolated lesion,mild or moderate TV disease does not need to betreated. Mild or even moderate TR may be observedusing current echo-Doppler techniques in normalsubjects. In the absence of structural changes suchas annular dilatation or leaflet disruption, suchlesions are not known to progress. On the otherhand, severe TV disease results in enlargement ofright atrium and right ventricle and increase in rightatrial and systemic venous pressures. If untreated,right ventricular dysfunction with reductionin cardiac output develop first with exerciseand subsequently at rest. This is accentuated bydevelopment of atrial fibrillation. In addition,chronic hepatic congestion results in fibrosis anddevelopment of cardiac cirrhosis. The liver functiontests become increasingly abnormal. Progressivedilatation of right heart chambers brings aboutprogressive annular dilatation, worsening severityof regurgitation. Thus, chronic severe regurgitationoften begets more regurgitation. For isolated severeTV disease, intervention should be considered asearliest signs of right ventricular and/or hepaticdysfunction develop.
The rules governing management are differentwhen moderate TV dysfunction is associated withother valvular or myocardial disorders. The timing ofintervention is generally dictated by considerationsrelating to accompanying left heart disease.Approximately 40% of patients exhibit regression ofTR following mitral valve surgery, with reduction inpulmonary hypertension. Since it fails to regress innearly 60% of patients, it is a recommended practice to treat tricuspid lesion more aggressively during themitral valve surgery.17
How to Treat?
Primary or intrinsic TV disease with severedysfunction nearly always requires surgery, with thepossible exception of rheumatic tricuspid stenosiswhich may be approached by percutaneous balloonvalvuloplasty.
Secondary or functional TR offers a wider arrayof options:
- Medical treatment: TR secondary to pulmonaryhypertension may be treated by medical managementof underlying etiology, when feasible. Thus,appropriate treatment of myocarditis or depressedleft ventricular function may result in ameliorationof functional TR. Similarly, improvement in lungfunction in chronic obstructive lung disease orappropriate control of sleep apnea may improve theassociated TR. It is worth emphasizing that functionalTR may be dynamic, being load dependent. Intensivemedical treatment of heart failure may improvedramatically the severity of TR. This is especiallyrelevant when a patient is undergoing surgery of leftheart disease (such as mitral or aortic valve disease)following intensive medical treatment of heartfailure, such that the most recent echocardiogrammay fail to show significant TR. In this setting evenif the TR is mild to moderate, it will require surgicaltreatment.
- Surgical treatment: Rheumatic tricuspid stenosisis nearly always associated with rheumatic mitralvalve disease. Successful mitral and TV repair maybe carried out, although long term results are poor.Mitral valve replacement with TV replacement maybe considered in patients unwilling to entertaina risk of reoperation. These patients will require mechanical prosthesis, being in a younger agegroup.
- Tricuspid regurgitation: since the most commonlyobserved TR undergoing surgery is functional orsecondary to mitral, aortic or ischemic heart disease;the surgical approaches will be considered in somedetail. Significant TR is often a marker of adverseoutcome.18,19 A variety of techniques for valve repairhave been used over the years. These fall broadlyinto 2 categories; suture techniques and annuloplastytechniques.
Suture techniques:
- De Vega purse string repair: since its inceptionin 1972, this technique has been extensively usedlong-term with considerable success.20 However, long-term follow-up studies reveal a significantly higherrecurrence rate as compared to use of annuloplastyring or band.5 The early results (up to 6 months) aregood, such that one would recommend its use incases where a rapid and sustained fall in pulmonaryartery pressure is likely to result following mitralvalve surgery. It is a more practical and economicalapproach in developing countries with high incidenceof rheumatic mitral disease being operated on atearly age (Table 3).5
- Suture plication of posterior leaflet: thisapproach has been used in some cases with extremeannular dilatation, but generally in combinationwith annuloplasty.
Annuloplasty techniques:
There is a growing body of evidence to supportimproved outcome and durability of TV repair usingannuloplasty ring. Tant et al reported freedom fromrecurrent TR in those receiving rings was 82 (5%) at15 years as compared to 39 (11%) (P=.0003) in repairswithout use of a ring. They also observed improvedlong term survival as well as event free survival forthose with TV repair with ring. McCarthy et al alsoreported a higher rate of failed TV repair withoutuse of rings. They observed 30% of patients with DeVega procedure had severe regurgitation at 8 yearsas compared to none with ring annuloplasty.5 A variety of annuloplasty rings and bands have beenused:
- Peri-Guard annuloplasty consists of customizedsemicircular annuloplasty using bovine pericardium.A high rate of early and late recurrence of TR hasbeen reported. This approach is not favored at thepresent time.
- Carpentier ring devised for the TV introducedmore than 30 years ago has been extensively used.This semi-rigid ring has had excellent early and lateoutcome. Special care has to be taken to avoid injuryto the AV node.
- Duran flexible ring has been proposed in order topreserve the normal annular function of dilatation indiastole and reduction in systole. Good early and lateoutcome has been reported using the flexible ring.
- Annuloplasty bands or incomplete rings areused to avoid risk of AV node injury. A partial ringspecially devised with knowledge of 3-dimensionalgeometry of the TV (MC3 ring) has been introducedwith promising early and mid-term outcome.
- Edge to edge annuloplasty technique consistingof stitching together free edges of the tricuspidleaflets ton produce a clover shaped valve has beendescribed. 21,22
Tricuspid valve replacement: although moststudies have reported a better early and long termoutcome with valve repair, there are some caseswith marked distortion of the annulus and severeleaflet tethering in which valve replacement maybe necessary. Generally bioprosthetic valves arepreferred, since valve thrombosis and infectionfollowing mechanical valve replacement are distinctrisks.23 Some studies have shown no significantdifference in long term outcome between tissueand mechanical valves.24-26 Residual regurgitation following TV replacement is lower than after valverepair; however, the perioperative midterm survivaland event free survival is better with valve repair(Table 4).4
The 2006 American College of Cardiology /American Heart Association (ACC/AHA) Guidelinesfor management of patients with valvular heartdisease pertaining to TV and pulmonary valve aresummarized in tables 5 and 6.27-29
Surgical Treatment of Primary Tricuspid ValveRegurgitation
Rheumatic Valve Disease
The surgical options include valve repairtechniques similar to those employed for rheumatic mitral valve disease. In rare cases with extremefibrotic distortion of the valve, TV replacement mayneed to be considered. In a study of 328 patientsfollowed over a mean 8.7 years, in-hospital mortalitywas 7.6% and late mortality was 52.1%. Valve repairhad a more favorable outcome.30
Ebstein's Anomaly
TV repair may be feasible in milder cases, althoughthe majority requires valve replacement. Good longterm outcomes and survival are reported. A studyexamined outcome of 40 consecutive patients atone center. The valve was repaired in 18 patients,in 12 in association with cavo-pulmonary shunt.Twenty-two underwent replacement, 11 with cavopulmonary shunt. There were 2 postoperativedeaths and 5 late deaths during follow-up of 6.7 (4.8) years. Arrhythmias were the most commonlate complication.31 The experience reported fromthe Mayo Clinic in 539 patients showed survival at5, 10, 15, and 20 years of 94%, 90%, 86%, and 76%respectively. Thirty-six percent experienced atrialfibrillation or flutter and 27% had endocarditis.28
Carcinoid Heart DiseaseSymptomatic patients with severe TV dysfunctiondespite treatment with somatostatin analoguesgenerally require valve replacement. Surgicalintervention, aside from relief of symptoms, is alsocredited with improved survival in this lethal disease.Balloon valvuloplasty has been used in rare caseswith predominant tricuspid stenosis. This representsa high risk surgical group.29
Infective Endocarditis
Infection of the tricuspid is commonly relatedto intravenous drug abuse and poses a significantchallenge in management. Early cases may undergosuccessful valve repair with resection of vegetation,focal leaflet resection and annuloplasty. However,the large majority have significant valve destructionand are candidates for valve replacement. Thechances of reinfection in drug addicts is considerableand medical follow-up is likely to be sporadic.Hence, tricuspid valvectomy has been utilized withgood initial results, since the resulting TR in absence of pulmonary hypertension is hemodynamicallywell tolerated. However, the long-term results arediscouraging. Replacement with bioprosthesis maybe preferred despite young age of the patient, owingto lack of reliance on oral anticoagulation therapy inthis group of patients.32
Cleft Tricuspid Valve
Most adult patients presenting with cleft TV havemilder pathology and are successfully repaired.Younger patients with extremely malformed valvesmay require valve replacement.
Iatrogenic Tricuspid Regurgitation
TR associated with pacemaker trauma is generallyamenable to valve repair. Rare cases with secondaryfibrotic changes may require valve replacement.
PULMONARY VALVE DISEASE
The overwhelming majority of lesions resultingin pulmonary valve disease are either congenital ora consequence of surgical treatment of congenitallesions. The acquired conditions are rare. Generally,disorders affecting right ventricular infundibulum,pulmonary root and artery are considered togetherwith the pulmonary valve. The etiologies ofpulmonary valve disease are listed in Table 7.
Clinical Presentation
Symptoms: these may result from reduced cardiacoutput in advanced cases with right ventriculardysfunction. Dynamic infundibular stenosis inpresence of ventricular septal defect may bring aboutcyanotic spells observed in children with Tetrologyof Fallot.
Physical signs: bedside examination may provideimportant clues. Pulmonary valve stenosis isassociated with characteristic auscultatory findingsdepending on severity. Mild stenosis is characterizedby a systolic ejection click and short early systolicmurmur. With progressive severity, the murmur getslouder, longer and peaks later in systole. The ejectionclick is often more prominent in expiration. Thisseemingly paradoxical behavior of the pulmonaryejection click is explained by an inspiratory increasein right ventricular end-diastolic pressure, whichopens the valve in late diastole and, hence, causesabsence of systolic ejection click during inspiratoryphase. Thus, ejection click may be absent in the mostsevere stenosis where right ventrucular end-diastolicpressure is consistently above the pulmonaryarterial pressures. The behavior of the second heartsound is also of diagnostic importance. In mildercases the pulmonary component of second heartsound (P2) is delayed, but retains further wideningwith inspiration. As stenosis increases in severity,the pulmonary component becomes softer and themurmur in the very severe cases spills past aorticcomponent and the pulmonary component isinaudible.
Clinical assessment of pulmonary regurgitation(PR) is often more challenging. A high-pitcheddiastolic murmur following a prominent P2 may beevident in patients with PR secondary to pulmonaryhypertension. This murmur is often described as the Graham Steell murmur and may be erroneouslyinterpreted to indicate aortic regurgitation as theyboth may be heard best along the left sternal border.Mild or even moderate PR may be present withoutan audible murmur.
Clinical assessment of infundibular pulmonarystenosis (PS) reveals a systolic murmur peaking inlate systole and well-preserved, but delayed, P2.
Clinical assessment of supravalve PS often providesdiagnostic clues. The murmur is often prolongedthrough systole and may spill past P2. The murmuris audible in axillary region and over the back. It isoften soft and may be easily heard after cessation ofrespiration.
Laboratory Diagnosis
Electrocardiography
Twelve lead ECG provides valuable clues in thediagnosis of pulmonary valve disease. The featuresof right ventricular hypertrophy and right atrialenlargement are commonly observed. Severe rightventricular hypertrophy and strain often indicatesupra-systemic right ventricular pressure.
Chest x-Ray
A routine PA and lateral chest x-ray also yielduseful clues. The pulmonary trunk is dilated in pulmonary valve stenosis. Reduced pulmonaryarterial vasculature indicates severe stenosis. Thepulmonary trunk is not dilated in infundibularstenosis, or in pulmonary atresia. Signs of rightventricular enlargement may be present.
Echocardiography
The echocardiogram provides diagnostic andquantitative assessment of pulmonary valve stenosis,infundibular PS, and PR. The pulmonary valvemorphology shows doming and incomplete openingin presence of pulmonary valve stenosis. Althoughthe valve cusps are normal in infundibular stenosis,a characteristic midsystolic closure and prominentpresystolic "a" wave are often diagnostic clues.The pulmonary artery and branches are dilated inpulmonary hypertension, idiopathic pulmonaryartery dilatation and severe PR. In rare cases ofpulmonary valve endocarditis, a mobile vegetationmay be observed. Hypertrophied and dynamicright ventricular infundibulum are characteristicfor infundibular stenosis, be it congenital orassociated with hypertrophic cardiomyopathy.The spectral Doppler and color-flow Dopplerreveal high-velocity turbulent flow in the mainpulmonary artery in patients with pulmonary valvestenosis, and a late-peaking, high-velocity flow withturbulence in the right ventricular outflow tract isnoted in infundibular PS. Trivial or mild PR arenormal findings in most children, as well as adults.However, moderate and severe regurgitation areassociated with right ventricular volume overloadand subsequent dilatation and dysfunction. ThePR velocity waveform provides a unique insightinto pressure difference between pulmonary arteryand right ventricle during diastole. Because rightventricular diastolic pressure equilibrates with rightatrial pressure in absence of tricuspid stenosis, anestimation of pulmonary arterial diastolic pressureis obtained using the end-diastolic velocity of PRand size of the inferior vena cava, which is used toestimate right atrial pressure (Table 6).27
Cardiac Catheterization and Selective Angiography
Diagnostic right-heart catheterization isuseful to measure pulmonary artery pressuresand pulmonary wedge pressure, and to calculatepulmonary vascular resistance. These are usefulto differentiate and quantify precapillary andpostcapillary pulmonary arterial hypertension.Although quantification of pulmonary valvestenosis is generally made using echo Dopplermethods, catheter-based measurements beforeand after pulmonary balloon valvotomy are used to evaluate successful dilatation of the stenoticpulmonary valve. Selective angiography is less usefulfor diagnostic or therapeutic interventions.
Treatment
Medical Treatment
The only medical treatment is palliative for reliefof right heart failure with hepatic congestion andperipheral edema. No patient should get to thisstage since there are a number of interventional andsurgical options to treatment of pulmonary outflowdisorders. Palliative medical treatment in form ofbeta adrenergic blockers is used for cyanotic spellsin Tetrology of Fallot.
Interventional Treatment
- Pulmonary balloon valvuoplasty: this procedureis often carried out in childhood even in absence ofsymptoms. Patients with peak Doppler gradientsin excess of 40 mm Hg are generally suitablecandidates for percutaneous balloon valvuoplasty.The procedure is highly successful in patients withpulmonary valve stenosis, with marked decrease intransvalvular gradient and increase in pulmonaryartery pressures.33 There is also normalizationof right ventricular end diastolic pressures andregression of hypertrophy. Sudden reduction ingradient may lead to subvalvular stenosis requiringthe use of beta adrenergic blocking agent. This isobserved infrequently and only in those with severepulmonary valve stenosis. The procedure may resultin rare complications including perforation, cardiactamponande or sudden cardiac arrest. Anothercomplication related to use of larger diameterballoons is occurrence of PR.34,35 Although initially mild or moderate, this may progress with time andrequire future interventions.
- Percutaneous pulmonary valve replacement.Following the first successful human percutaneousimplantation of a catheter based stent valve inpulmonary position by Bonhoeffer in 2000, thefirst series included 8 patients with surgicallyrepaired congenital pulmonary valve disease.36 A later experience in 59 patients was reported in2006, using Melody bovine jugular vein valve37 (Medtronic; Minneapolis, Minnesota). More recentexperience in 155 patients illustrated the impact ofevolving technology and operator learning curve.38 A prospective multicenter clinical trial confirmssafety and efficacy of implantation of the Melodytranscatheters pulmonary valve in patients witha dysfunctional right ventricular outflow tractconduit.39 This technique has also been used inpulmonary homograft stenosis after Ross operation.
The use of both the percutaneous valve replacementand of placement of stents are promising interventionsin experienced hands.
Surgical Treatment
Surgical valvotomy for severe pulmonary valvestenosis is a well established procedure with aconsiderable track record. Early complicationincludes subvalvular stenosis, requiring betaadrenergic blocker as treatment of choice. Itimproves spontaneously as hypertrophy regresses.Additional complication is development of PR,which may progress in severity with late sequelae.
The surgical procedures designed to reconstructthe outflow tract using conduit are commonly used.These have late complications including stenosis ofthe conduits and development of progressive PR.
ABBREVIATIONS
PASP: pulmonary artery systolic pressure
PISA: proximal isovelocity surface area
PR: pulmonary regurgitation
PS: pulmonary stenosis
TEE: transesophageal echocardiography
TR: tricuspid regurgitation
TV: tricuspid valve
Correspondence: P. M. Shah, MD, MACC,
Chair, Medical Director, Hoag Heart Valve Center,Hoag Memorial Hospital Presbyterian,
Newport Beach, CA, USA
E-mail: pshah@hoaghospital.org