Keywords
INTRODUCTION
In recent years, several studies have shown thatright ventricular function is an important predictorof survival in patients with congenital heart disease,pulmonary hypertension and heart failure (HF).1-3 In 2006, the National Heart, Lung and Blood Instituteidentified right ventricular function and failure as apriority for research in cardiovascular disease.4 Rightventricular (RV) function may be impaired in several conditions such as right ventricular myocardialinfarction (RVMI), acute pulmonary embolism, leftheart disease, parenchymal lung disease, pulmonaryvascular disease or congenital heart disease.5-10 Our understanding of right heart failure (RHF) hasconsiderably improved in the last 2 decades. In thisreview article, our objective is to critically presentthe evidence that underlies the management ofRHF. We also discuss important perils and pitfallsthat may help in the management of patients withRHF.
METHODS
A systematic review of the literature using PubMedand the latest issue of the Cochrane Central Registerof Controlled Trials was performed for studiesconducted between January 1975 and January 2010.11,12 The search was focused on both observationaland randomized controlled trials with a minimumof 5 subjects. Book chapters, meta-analysis, reviewarticles, and editorials were also scanned. The searchterms used included, HF, right heart, right ventricleand specific therapies, eg, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensinreceptor blockers (ARB), sildenafil, hydralazine,pacemaker, defibrillators, and cardiothoracic surgery.Searches using individual brand names of medicationswere also conducted.
All studies identified through our searches wereassessed by 2 reviewers and consensus was requiredfor inclusion in the review. Data extraction wasdone independently by the 2 investigators usinga pre-defined form. The following data wereextracted: methods (study design, method ofrandomization, concealment of allocation, blindingof the investigators, inclusion and exclusion criteria),populations (sample size, age, and sex), participantcharacteristics, etiology of heart disease, intervention(agent, dose, timing and duration of therapy, andother medications), control (participants, agent, anddose), and outcomes measures. The main studiesrelevant to the management of RHF are summarizedin table format that summarize study design andmain outcome measures. When relevant, we alsoreport recommendations based on the most recentlypublished consensus or guideline statement.1,3,13 When appropriate, we also quote in parenthesisthe recommendations of the American College ofCardiology (ACC)-American Heart Association(AHA) or European Society of Cardiology (ESC).The recommendations are classified according tothe strength of the recommendation (I, IIa, IIb, III[contraindicated]) and the level of evidence (A, B, C[expert consensus]).
DEFINITION OF RHF AS A SYMPTOMATICAND PROGRESSIVE DISORDER
RHF is defined as a complex clinical syndromethat can result from any structural or functionalcardiac disorder that impairs the ability of the rightheart to fill or eject appropriately.1 The cardinalclinical manifestations of RHF are: a) fluidretention manifested as peripheral edema or ascites; b) decreased systolic reserve or low cardiac outputsyndrome, which may present as exercise intolerance,fatigue or altered mentation; and c) atrial orventricular tachyarrhythmias. Functionally, theaffected right ventricle may be in the subpulmonary(usual) or systemic position (in congenitallycorrected transpositions of great vessels (L-TGV) orD-TGV following an atrial-switch repair). Patientsmay present with a clinical picture of biventricularfailure or predominantly RHF.
When considering RHF as a progressivedisorder, patients with asymptomatic ventriculardysfunction are considered to be in the earlystages of RV failure.1 Analogous for the stagingproposed for left heart failure (LHF), patients mayprogress from being at risk of RHF (stage A), toasymptomatic RV dysfunction (stage B), to RHF(stage C) and finally refractory RHF (stage D).1 Itis also practical to divide RHF as to whether it isacute or chronic.
ETIOLOGY AND PATHOPHYSIOLOGY OF RHF
The clinical syndrome of RHF may result fromdisorders of the myocardium, the pericardium, endocardium, pulmonary vasculature and pulmonaryparenchyma (Table 1). LHF represents the mostcommon cause of RHF. Table 2 summarizes theclassification of pulmonary hypertension, a commoncause of RHF.
Following initial myocardial stress or injury,several factors may contribute to progression ofRV failure, including the timing of myocardialstress (adult period > pediatric), type of stressor(pressure overload > volume overload), andmyocardial ischemia, as well as neuro-hormonaland immunologic activation.7,8 At a molecular level, maladaptative RV remodeling has beenassociated with a switch in contractile proteinisoforms, alteration in cardiac metabolism, alterations in enzymes and ion channels involvedin myocyte excitation-contraction coupling, matrixremodeling and neurohormonal and cytokineactivation.14-21 Among the neurohormones involved in RV failure, evidence is stronger forangiotensin-II, catecholamines and natriureticpeptides.19-21 Recent studies also demonstrate thatspecific pathways may be selectively involved inRV remodeling. Urashima and colleagues haveshown that genes may be differentially regulatedin the pressure overloaded RV as compared tothe pressure overloaded left ventricle (LV). Thedifferentially expressed genes were involved inthe Wnt signaling pathway, apoptosis, migrationof actin polymerization and processing of theubiquitin system.22 The differential expressionof genes in the right heart and left heart is notsurprising in view of the different embryologicalorigin of the RV and LV and their differentphysiological environments.23,24
Ventricular interdependence also plays an essentialpart in the pathophysiology of RHF. Althoughalways present, ventricular interdependence is mostapparent with changes in loading conditions such asthose seen with volume loading, respiration or suddenpostural changes.25 Ventricular interdependence helps maintain hemodynamics in early stages ofRHF. Experimental studies have shown that in theabsence of a dilated RV, LV systolic contractioncontributes 20%-40% of RV systolic pressuregeneration.25,26 Diastolic ventricular interdependencecontributes to the development of LV systolicdysfunction in patients with RHF. RV enlargementor increased afterload may shift the interventricularseptum and increase pericardial constraint on theleft ventricle; both of these changes may alter leftventricular geometry and decrease LV preload andcontractility (Figure 1).25,27 Compression of the leftmain coronary artery by a dilated main pulmonaryartery, which is occasionally observed in pulmonaryarterial hypertension (PAH), may contribute to LVdysfunction.28 Tricuspid regurgitation and ongoingischemia may also contribute to the progression ofRHF.
Figure 1. Pathophysiology of right heart failure. LM: left main coronary artery; LV: left ventricular; R to L: right to left; TR: tricuspid regurgitation; V interdepend.: ventricular interdependence; V/Q: ventilation-perfusion. Adapted from Haddad et al.8
DIAGNOSTIC EVALUATION OF PATIENTSWITH RHF
The goals of the initial evaluation of patients withRHF are to better characterize its etiology, severityand functional status, the presence and extent of end-organ damage (renal dysfunction, liver dysfunction) and the presence of associated conditions. In patientswith RHF, physical examination often reveals lowerextremity edema, jugular venous distension and aparasternal holosystolic murmur compatible withtricuspid regurgitation. Cyanosis may be observedin patients with right to left shunting or severe lowcardiac output.
Echocardiography plays a key role in the diagnosisof right heart disease. Signs of right heart disease onan echocardiogram can include RV enlargement,RV systolic dysfunction, tricuspid regurgitation,and pulmonary hypertension, congenital heartdefects, valvular heart disease, or left heart disease.Magnetic resonance imaging (MRI) is becoming thegold standard for evaluating right heart structureand function and is particularly useful in patientswith complex congenital heart defects (eg, Ebstein'sanomaly, hypoplasic RV), in patients in whomprecise quantification of valvular regurgitation isimportant, and for planning of a complex surgery orprocedure or for research purposes.8 Recent studiesusing MRI have also demonstrated the prognosticvalue of RV end-diastolic volumes and pulmonarycompliance assessed by MRI in pulmonary arterialhypertension.29,30 Angiography by MRI, computedtomography-angiography or heart catheterizationmay be of particular value in excluding chronicthromboembolic pulmonary disease, in assessingcomplex arterio-venous malformations orcongenital heart defects. Right heart catheterizationis an important part of the evaluation of right heartdisease. Indications for right heart catheterizationinclude assessment of pulmonary vascular resistanceor impedance, pulmonary pressures, cardiac outputshunt fraction, and pulmonary vasoreactivity.Exercise testing is also very useful in objectivelyassessing clinical deterioration in patients with PAHor congenital heart disease. Caution is, however,advised in performing maximal exercise testing inpatients with severe pulmonary vascular disease(contraindicated in the recent AHA consensus oncongenital heart disease).3
Obtaining baseline renal and liver function tests,albumin, uric acid levels as well as B-type natriureticpeptide levels may be of particular interest indetermining prognosis of right heart disease.31-37 In patients with severe hypoalbuminemia, proteinlosing enteropathy should be excluded with the assayof stool alpha-1 antitrypsin. Electrocardiography ispart of the routine evaluation and allows assessmentof cardiac rhythm, QRS duration or the presence ofatrio-ventricular conduction block.
Other studies should be individualized dependingupon the suspected etiology of RHF. In patientswith PAH, a ventilation perfusion scan, pulmonaryfunction tests, overnight oximetry, serologies for HIVand connective tissue disease (eg, antinuclear antibody test, ANA) are often routinely obtained. Stool alpha-1antitrypsin are often obtained to rule out proteinlosing enteropathy. Lung or heart biopsy is rarelyindicated in patients with isolated right heart disease.Genetic counseling should be pursued in patientswith congenital heart disease or arrhythmogenic rightventricular dysplasia (ARVD). Depending on theetiology and severity of RHF, patients are followedat varying intervals (usually 3 months to 1 year).The recent guidelines for congenital heart diseaseand PAH offer individualized timing for follow-updepending on conditions.3
MANAGEMENT OF RHF
Overview of the Management of RHF
The most important aspect of managingRHF is tailoring therapy to its specific cause. Incontrast to patients with chronic ischemic or nonischemic cardiomyopathy, patients with RHFoften have significantly abnormal afterload (eg,pulmonary hypertension) or valvular heart disease(acquired or congenital pulmonary or tricuspiddisease). It is therefore not surprising that theselected therapy should primarily target the causeof RHF. In managing patients with RHF, it isalso useful to divide the syndrome into 4 clinicalcategories: biventricular failure, systemic RVfailure, predominant sub-pulmonary RV failure,and hypoplasic RV syndrome.3 The management ofpatients with hypoplasic RH is beyond the scope ofthis review and the reader is referred to the recentconsensus statement of Warnes and colleagues.3
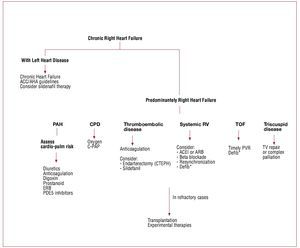
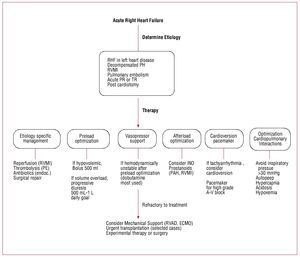
The physiological goals of RHF treatment includeoptimization of preload, afterload and contractility.Sodium and fluid restriction and judicious use ofdiuretics all help optimize RV preload. Clinically,optimal preload may be defined as the preloadthat results in optimal cardiac output withoutcausing renal dysfunction. Although it is oftenperceived that patients with RHF require higherlevels of filling pressure, the majority of patientsmay have an optimal preload with normal rightatrial pressure (<6 mmHg). Small clinical studiesalso suggest that resynchronization therapy may bebeneficial in selected patients with RHF.38-41 As will be discussed, only small studies suggest a beneficialrole for beta blockade or ACE inhibitors in RHF;these results have not been confirmed by largerstudies. Primary prevention of sudden death usingdefibrillators is mainly recommended in patientswith arrhythmogenic RV dysplasia and tetralogy ofFallot.42,43 In the setting of acute RHF, every effort should be made to avoid systemic hypotension, as this could lead to myocardial ischemia and further hypotension. Figures 2, 3, and 4 summarize themanagement of chronic and acute RHF and PAH.
Figure 2. Management of chronic right heart failure syndrome. CDP: continuous distending pressure; C-PAP: continuous positive airway pressure; CTEPH:chronic thomboembolic pulmonary hypertension; Defib.: defibrillator; ERB: endothelin receptor blockers; PAH: pulmonary arterial hypertension; PDE5: phosphodiesterase-5; PVR: pulmonary valve replacement or repair; RV: right ventricle; TV: tricuspid valve; TOF: tetralogy of Fallot. Adapted with permission fromHaddad et al.9
Figure 3. Management of acute right heart failure. ECMO: extra corporeal membrane oxygenation; Endoc.: endocartidis; PE: acute pulmonary embolism; PH:pulmonary hypertension; PR: pulmonary regurgitation; RHF: right heart failure; RV: right ventricle; RVAD: right ventricular assist device; RVMI: right ventricularmyocardial infarction; TR: tricuspid regurgitation; WHO: World Health Organization. Adapted with permission from Haddad et al.9
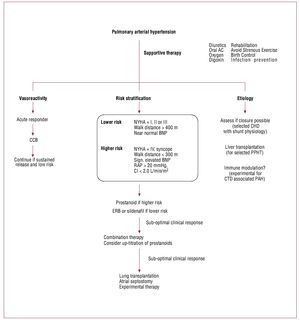
Figure 4. Management of pulmonary arterial hypertension. Treatment of patients with pulmonary arterial hypertension depends on pulmonary vasoreactivity,risk stratification and selected etiology. AC: anticoagulation; CCB: calcium channel blockers (amlodipine, felodipine or diltiazem); CHD: congenital heartdisease; CI: cardiac index; CTD: connective tissue disease; ERB: endothelin receptor blockers; NYHA: New York Heart Association; PAH: pulmonary arterialhypertension; PPHT: porto-pulmonary hypertension; RAP: right atrial pressure. Adapted from McLaughlin et al.2
Evidence Underlying the Management of RHF
Compared to the evidence supporting themanagement of LHF, the management of RHFis not well supported by randomized controlledtrials. Furthermore, clinical trials in patients withRHF have not been powered for mortality endpoints. Among patients with RHF, the evidence isbest established for patients with PAH.1-3 In PAH it may, however, be difficult to distinguish whetherthe beneficial effects of therapy are due to changes in pulmonary vasculature or RV specific effects;we therefore often consider the effects of PAHtherapy in the context of the cardiopulmonaryunit. In patients with congenital heart disease, theeffects of therapy have not been consistently studiedacross functional class severity (New York HeartAsssociation [NYHA] functional class).
Because the prevalence of RHF is relativelysmall compared to the prevalence of LHF, findingappropriate surrogate end-points has been animportant focus of research.44,45 Surrogate end-pointsbeing considered include exercise capacity, clinicalworsening, ventricular remodeling or measures ofvascular impedance in pulmonary hypertension.
General Preventive Measures
Referral to a congenital heart disease orpulmonary hypertension specialist when appropriateis recommended in patients with pulmonary arterialhypertension or complex congenital heart disease.2,3 This measure will help avoid any delay in timelyintervention such as cardiac surgery, percutaneousclosure of a cardiac defect or percutaneous valvereplacement or initiation of PAH specific therapy.2,3
Prevention or early recognition of RHFdecompensation is key in managing RHF. Factorsthat may lead to volume overload include non-compliance with sodium (<2 g daily) or fluidrestriction, non-compliance with medications or use of nonsteroidal anti-inflammatory drugs or nondihydropyridine calcium channel blockers. Patientswith significant PAH or severe RHF should alsobe advised against pregnancy, as it is associatedwith increased maternal and fetal mortalityrate.2,3 Prevention of infection with influenza andpneumococcal vaccination is also recommended,as is prophylaxis against bacterial endocarditis inpatients with mechanical valves, previous infectiousendocarditis or in patients with selected congenitalheart defects.2,3
Recent studies have shown that supervisedcardiac rehabilitation for 8 weeks led to a significantimprovement in 6 minute walk test (6MWT) inpatients with PAH (mean difference of 111 m compared to placebo).46 Interestingly, the absoluteimprovement was greater than any change in 6MWTwith PAH targeted therapy. Patients are howeverstill advised against intense exercise or travel toaltitudes above 5000 feet.
ETIOLOGY-BASED MANAGEMENT OF RHF,THE MAIN TARGET
Biventricular failure is managed following theguidelines of the AHA/ACC or ESC for managingpatients with chronic HF.1 Patients with biventricular failure benefit from beta blockade and ACEinhibition or angiotensin receptor blockers. Recentstudies have shown that sildenafil may improvepulmonary hemodynamics, exercise capacity andendothelial function in patients with chronic systolicHF (Table 3).47-51 Whether patients with left HFand evidence of right heart dysfunction may derivegreater benefits from sildenafil still remains to beproven.
In patients with ST elevation myocardial infarctioninvolving the right ventricle, early reperfusionshould be achieved as early as possible.52 Althoughthe RV usually recovers following acute myocardialinfarction, reperfusion therapy has also been shownto improve RVEF and reduce the incidence ofcomplete heart block.53,54 Maintenance of atrioventricular synchrony, correction of bradycardiaand maintenance of hemodynamic stability withappropriate volume loading or inotropic supportare also recommended.52
In patients with acute hemodynamicallycompromising pulmonary embolism (systolicblood pressure <90 mmHg or a drop in systolic blood pressure of ≥40 mmHg from baseline),evidence supports the use of thrombolytic agents(alteplase).55,56 Although many clinicians advocatethrombolytic therapy in patients with evidenceof RV dilatation and dysfunction withoutsystemic hypotension, the indication has notgained widespread acceptance and remains highlycontroversial.55,56 The controversy arises from thefact that clinical trials did not stratify patientsaccording to RV size and function, although isolatedRV dysfunction is a clear prognostic factor ofoutcome. In patients with chronic thromboembolicdisease, pulmonary endarterectomy may decreasepulmonary pressures to near normal and reverseRHF.57,58 Because pulmonary endarterectomy maybe life saving, pulmonary angiography, usuallyusing computed tomography scan, is a routine partof clinical investigation.
The treatment of PAH has evolved tremendouslyin the last 20 years. The ACCF/AHA 2009 ExpertConsensus Document on Pulmonary Hypertensionhas recently summarized its recommendations onpulmonary hypertension.13 Figure 4 summarizes the current management of PAH. The majority oftreatments for PAH were approved on the basis ofan improvement in 6MWT. Because of the smallnumber of patients with PAH, powering the studiesfor mortality outcomes will prove to be difficult.Also, more attention is being focused on the effectsof therapy on both the pulmonary vasculature andthe heart (cardio-pulmonary unit). For example,while both endothelin receptor blockers (bosentan)and sildenafil improve 6MWT, recent studiessuggest that sildenafil may also have positive inotropiceffects on the RV.59 Whether these differences willtranslate in to beneficial long-term effects remainsto be proven. Anticoagulation is also recommendedin patients with PAH based on several observationalstudies or sub-analysis of randomized trials (Table 4).60-63 Digoxin may lead to clinical improvement,but the evidence favoring its use is based onimprovement in acute hemodynamic profile andnot long-term effects (Table 5).61,63-66 An interesting recent advance is the demonstration that prostanoidtherapy may allow sufficient reversal of pulmonaryvascular disease to allow closure of an atrial septaldefect.13
In patients with tetralogy of Fallot followinginitial repair, pulmonary valve replacement isrecommended in patients with symptomaticpulmonary regurgitation (I, B) or in asymptomaticpatients with moderate to severe RV enlargement(IIa, C), moderate to severe RV dysfunction (IIa, C),or moderate to severe tricuspid regurgitation (IIa, C). Other indications include new onset sustainedatrial or ventricular arrhythmias or residual significant stenosis (peak RV outflow track gradient >50 mmHg by echocardiography or 70% systemicvalues; (IIa, C)) or residual ventricular septal defect,with a left-to-right shunt greater than 1.5:1 (IIa, B).3 Coronary artery anatomy, specifically the possibilityof an anomalous anterior descending coronary arteryacross the RV outflow track, should be ascertainedbefore any operative intervention (I, C).3
In patients with congenitally correctedtransposition of great arteries (L-TGA) or dextro-transposition of great arteries (D-TGA), the RVfunctions as the systemic ventricle. In both theseconditions, surgery or intervention is often consideredin the presence of significant atrio-ventricular(morphologically tricuspid) valve regurgitation,significant baffle leaks (D-TGA), significant orunrepaired defects, or conduit stenosis (please referto the recent guidelines of Warnes and colleagues formore complete information).3 The benefits of ACE-inbition or beta-blockade in patients with D-TGAor L-TGA is not well established (Tables 6 and 7).The studies addressing the question are small andunderpowered for mortality or clinical outcome.If beta-blockade is given, caution must be usedbecause of the risk of precipitating advanced atrioventricular block (especially in patients with preexisting sinus or AV nodal dysfunction).
In patients with Ebstein's anomaly, surgeryshould be considered in the presence of symptomswith deteriorating exercise capacity, (I, B), cyanosis(oxygen saturation less than 90%) (I, B), paradoxicalembolism, (I,B) or progressive RV dilatation orventricular dysfunction (I,B). The primary operation generally consists of closure of any interatrialcommunications; antiarrhythmia procedures such assurgical division of accessory conduction pathways,cryoablation of atrioventricular node reentrytachycardia, or Maze procedure; and tricuspid valvesurgery. The tricuspid valve is repaired when feasible,and tricuspid valve replacement is performed with amechanical or heterograft bioprosthesis when repairis not feasible or the repair result is not satisfactory.A right reduction atrioplasty is often performed.3
In patients with valvular pulmonary stenosis,percutaneous valvotomy is recommended inasymptomatic patients with a peak instantaneousgradient by Doppler greater than 60 mm Hg or amean Doppler gradient greater than 40 mm Hg ora symptomatic patient with a peak instantaneousDoppler gradient greater than 50 mm Hg or a meanDoppler gradient greater than 30 mm Hg. The RVusually remodels well after intervention, in theabsence of severe RV enlargement.
Recent studies also suggest that patients with flailtricuspid valves may benefit from earlier repair.Messika-Zeitoun and colleagues have demonstratedthat flail tricuspid valve is associated with a decreasedsurvival and a high incidence of HF, atrial fibrillationand need for valve replacement.67 At this time, centerswith low surgical mortality consider early interventionfor asymptomatic patients with flail tricuspid valve.
EVIDENCE UNDERLYING ACE INHIBITION,BETA-BLOCKADE, DIGOXIN ANDHYDRALAZINE THERAPY IN RHF
With the success of medical management of patientswith LHF, several researchers have investigatedwhether therapies proven to be beneficial in LHFmay be applied to RHF.68-80 Because most of thesestudies are underpowered for mortality outcomesand commonly underpowered for exercise capacity,definite conclusions are difficult to make at thistime.
A small study supports the use of digoxintherapy in patients with idiopathic pulmonaryhypertension.64 In the study of Rich and colleagues,intravenous digoxin therapy produced a modestincrease in cardiac output, as well as a significantreduction in circulating norepinephrine.64 At this time, no evidence clearly supports the use ofdigoxin therapy in patients with chronic obstructivepulmonary disease and associated RHF.65,66,81
Following initial enthusiasm associated with theuse of beta blockade in patients with systemicRV,73,74 a larger randomized controlled trial inpediatric patients with HF did not demonstrate abeneficial trend.71 A multicenter prospective trialon beta blockade in patients with PAH is currentlyongoing. In patients with tetralogy of Fallot, no benefit of the beta-blocker bisoprolol on exercisecapacity was demonstrated, although this wasonly studied in patients with NYHA functionalclass 1 or 2.82 Finally in patients with pulmonaryhypertension associated with liver disease (porto-pulmonary hypertension), a detrimental effecton hemodynamics was noted in a small studyof 10 patients.70 The use of ACE inhibitorsor angiotensin receptor blocker was also notassociated with any significant beneficial effectson exercise capacity or hemodynamics in patientswith systemic RV.75-80 Although the hydralazineand nitrate combination showed beneficial effectsin some patients with LHF, the use of hydralazinein PAH was associated with either detrimentaleffects or less beneficial effects than prostacyclintherapy (Table 8).83-89
NITRIC OXIDE THERAPY AND RHF
Initially, there was a lot of enthusiasmsurrounding the use of inhaled nitric oxide (iNO)in patients with RHF or pulmonary hypertension.This was based on the fact that iNO could provide selective pulmonary vasodilatation withoutcausing systemic hypotension or worseningventilation-perfusion mismatches. This enthusiasmwas, however, curbed by the practical difficultiesassociated with its administration, the developmentof alternative therapies, and the failure of studiesto show consistent benefits of iNO (Table 9). Aninteresting recent study has, however, showed thatiNO could lead to acute hemodynamic improvementwhen administrated to patient with RV myocardialinfarction complicated by cardiogenic shock.90 At this time, iNO is primarily used for acutevasoreactivity testing in PAH, and treatment ofpatients with acute RHF or severe hypoxemiafollowing lung transplantation (Table 9).49,90-101 Small reports also suggest that iNO may increaseleft filling pressures and precipitate pulmonaryedema in patients with concominant LHF.92
The role of prophylactic iNO in patients withpulmonary hypertension undergoing cardiacsurgery remains controversial. Ongoing studies areinvestigating the role of inhaled vasodilators such asmilrinone or sildenafil in preventing post-operativeRHF following cardiac surgery.102,103
PHOSPHODIESTERASE 5 INHIBITORS INPATIENTS WITH RHF OR PULMONARYHYPERTENSION
The use of phosphodiesterase-5 inhibitors in thetreatment of PAH has received much attention in thelast few years.47-51 PDE5 inhibitors have beneficialeffects on pulmonary vascular remodeling withminimal effects on the systemic vasculature (otherthan the penile circulation). Sildenafil has also beenshown to improve RV remodeling and contractility.While expression of phosphodiesterase 5 (PDE5)is minimal in the normal RV (where it is only expressed in the smooth muscle cells of the coronaryarteries), its expression is markedly increased inthe hypertrophied RV (as well as in the neonatalRV).59,104 Ongoing multicenter studies are currentlyunderway to assess the effects of sildenafil in patientswith diastolic HF.
INOTROPIC THERAPY
Inotropic therapy is indicated in patients withacute RHF and signs of low cardiac output. Amongthe inotropic or vasopressor agents, dobutaminehas been the most extensively studied in RHF.105,106 In RVMI, dobutamine increased cardiac index andstroke volume while maintaining preload.105 In PAH,dobutamine at doses of 2-5 mcg/kg/min increasecardiac output while decreasing pulmonary vascularresistance.106 The combination of dobutamine andiNO in pulmonary hypertension has also beenshown to increase cardiac index, decrease pulmonaryvascular resistance and significantly increase PaO2/FiO2 ratio.106 Dopamine use is often reserved forhypotensive patients, while milrinone is preferred inthe presence of tachyarrythmias. While epinephrineinfusion is commonly used in the intensive care unit,its specific effects in pulmonary hypertension havenot been well studied.
Levosimendan is a calcium sensitizer with inotropicproperties. Recent studies suggest that levosimendancould improve RV function or pulmonaryhemodynamics in patients with biventricular failureor ARDS.107-109 Future studies will determine its rolein managing patients with acute RHF.
MAINTENANCE OF SINUS RHYTHMRESYNCHRONIZATION THERAPY
Maintenance of sinus rhythm and heart ratecontrol is important in RHF. Advanced AVblock or atrial fibrillation can have profound hemodynamic effects in patients with acuteRHF or severe RV dysfunction. In patients withLHF, cardiac resynchronization therapy (CRT)has been shown to improve both survival andexercise capacity and is currently indicated inpatients with a QRS>120msec and evidence ofLV systolic dysfunction (LVEF<35%).1 Recentstudies also suggest that resynchronization maybe beneficial in patients with RHF (Table 10).38-41 In a multicenter international study, Dubinand colleagues demonstrated that CRT inpatients with RV dysfunction was associated withimprovement in RV ejection fraction (RVEF)in patients with either systemic or pulmonic RV(Table 10).38 Dubin and colleagues had previouslyshown that atrioventricular pacing in patientswith RBBB and RV dysfunction augments RVand systemic performance.40 Multicenter studiesof resynchronization in patients with PAH arecurrently planned.48
PREVENTION OF SUDDEN DEATH ANDDEFIBRILLATOR THERAPY
The mechanisms of sudden death inpatients with RHF vary depending on itsetiology. Ventricular tachycardia/RV fibrillation, pulmonary embolism, pulmonary hemorrhageor mechanical or electrical complications inRVMI may all contribute to sudden death.Optimal management such as revascularization,treatment of pulmonary hypertension, andcorrection of congenital defects can decreasethe incidence of sudden death.
Prediction of sudden death in RV failure is difficultand criteria have mainly been developed in patientswith arrhythmogenic RV dysplasia and tetralogyof Fallot.42, 43 The incidence of sudden death forthe adult tetralogy population can be estimatedfrom several large series to be on the order of 2.5%per decade of follow-up.3 Risk factors for suddendeath in tetralogy of Fallot include prolonged QRSduration (QRS>180 ms), or inducible ventriculararrhythmias using programmed ventricularstimulation during electrophysiology study.3 Because of the absence of long-term outcomestudies, primary prevention of sudden death usingimplantable defibrillators in tetralogy of Fallotremain center-specific.3 In patients with ARVD,primary prevention of sudden death is considered(IIa, C) in the presence of extensive disease, a familyhistory of sudden cardiac death or undiagnosedsyncope when ventricular tachycardia or ventricularfibrillation has not been excluded as the cause ofsyncope.110 In patients with PAH, prophylacticantiarrhythmic therapy is contraindicated and therole of defibrillator therapy for primary preventionof sudden death not defined.110
TRANSPLANTATION, ATRIAL SEPTOSTOMY,AND MECHANICAL SUPPORT
In patients with advanced refractory RHF,transplantation can be considered after exclusionof all reversible causes and careful consideration ofcontraindications. In appropriate patients with severepulmonary vascular disease, heart-lung or doublelung transplantation are considered.111 Because ofscarcity of organs, heart-lung transplantation isusually considered only in patients with congenitalheart defects and in patients in whom the physicianconsiders recovery of right heart function unlikely.Predictors of persistent RV failure after doublelung transplantation have, however, not been wellestablished at this time.
The observation of improved survival ofpatients with pulmonary hypertension and patentforamen ovale has led to the hypothesis that atrialseptostomy, which "decompresses" the RV andincreases right to left shunting, could be helpful insevere RV failure. The response to atrial septostomyin pulmonary hypertension is variable. At this time,atrial septostomy should be considered palliative.112
In patients with acute RHF refractory to medicaltreatment, mechanical support of the RV issometimes used as a bridge to transplantation or abridge to recovery. The most common indicationsfor RVAD use are severe RV failure after LV assist-device, RV failure after heart transplantation orRV failure after massive pulmonary embolism.113 Permanent implantation or "destination therapy" for chronic advanced RV failure has not beenstudied. Future studies will determine whethermechanical support using axial flow devices couldbe of benefit in patients with refractory RHF with acontraindication to transplantation.
PERILS AND PITFALLS
Management of RHF may be at the same timesimple and complex. In order to optimize patientcare, several pitfalls should be avoided. Among them,the most common ones include: a) ordering maximalexercise testing in patients with severe pulmonaryvascular disease; b) failure to exclude chronicthromboembolic disease as a cause of pulmonaryhypertension; c) delaying referral to a specializedcenter for appropriate surgical, interventional ormedical therapy; d) excessively volume loading apatient with acute RHF; or e) closing an atrial septaldefect in a patient with severe pulmonary vasculardisease. Caution should also be advised when usinginhaled nitric oxide or sildenafil in patients withsevere left ventricular filling pressures as increasedcardiac output can precipitate pulmonary edema.
EMERGING THERAPIES FOR RHF
In the next few years, several specific therapies forRHF are potentially emerging. Among therapies thatimprove energy utilization of the heart, metabolicmodulators are probably the most promising atthis time. Recent experimental studies show thatmetabolic modulation reverses maladaptative RVremodeling in rats with monocrotaline inducedPAH.16,114-117 Other potential new therapies include myosin activators, Na/K-ATPase inhibitors,adenosine or vasopressin antagonists or micro-RNA modulators.118 An interesting case report alsosuggests that tricuspid annuloplasty could play arole in managing patients with severe PAH.119
CONCLUSION
RHF is a complex clinical syndrome that presentswith lower extremity edema, ascites, decreasedexercise tolerance, or arrhythmia. Common causes ofRHF include right ventricular myocardial infarction,pulmonary embolism, congenital heart disease, andpulmonary hypertension from a variety of causes. Atthis time, advances in therapy have mainly been madein treatment of pulmonary arterial hypertension andsurgical repair of complex congenital lesions. Ongoingstudies will investigate the role of betablockade orcardiac resynchronization therapy in patients withPAH. Emerging new therapies may include metabolicmodulators or myosin activators.
ACKNOWLEDGMENTS
We want to acknowledge the educational support ofthe Vera Moulton Wall Center for Pulmonary VascularDisease at Stanford as well as the support of the Divisionof Cardiology of Stanford University.
ABBREVIATIONS
6MWT: 6-minute walk test
ARVD: arrhythmogenic right ventriculardysplasia
CO: cardiac output
CTEPH: chronic thromboembolic pulmonaryhypertension
ERB: endothelin receptor blockers
HF: heart failure
iNO: inhaled nitric oxide
LV: left ventricular
LHF: left heart failure
LVEF: left ventricular ejection fraction
MPAP: mean pulmonary arterial pressure
NYHA: New York Heart Association
PAH: pulmonary arterial hypertension
PCWP: pulmonary capillary wedge pressure
PDE5: phosphodiesterase-5
PVR: pulmonary valve replacement or repair
PVRI: pulmonary vascular resistance index
RAP: right atrial pressure
RBBB: right bundle branch block
RCT: randomized placebo controlled trial
RHF: right heart failure
RV: right ventricular
RVEDP: right ventricular end-diastolic pressure
RVEF: indicated right ventricular ejectionfraction
RVMI: right ventricular myocardial infarction
TGA: transposition of the great arteries
TOF: tetralogy of Fallot
TTPG: transtricuspid pressure gradient
Correspondence: F. Haddad, M.D.
Division of Cardiovascular Medicine, Stanford University School
of Medicine, Palo Alto, California, United States of America, 94305.
E-mail: fhaddad@stanford.edu