Left heart failure is the final manifestation of various heart diseases involving left ventricular dysfunction, such as ischemic heart disease.1 Due to treatment advances, the prevalence of left heart failure is growing, but its natural course is associated with a continual decrease in quality of life, rehospitalizations, and early mortality.2 Previous studies have shown that tight control of left atrial pressure is associated with improved ventricular function and functional class, suggesting that this strategy could improve prognosis.1
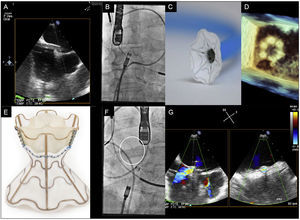
The V-Wave device (V-Wave Ltd, Or Akiva, Israel) is based on this concept and on past experience of the creation of interatrial shunts to treat patients with congenital heart disease or ventricular assist devices.3–5 The V-Wave device, which permanently reduces left atrial pressure, is composed of an hourglass-shaped nitinol frame with a polytetrafluoroethylene polymer coating on its left side and a valve with 3 bovine pericardium leaflets on its right side to prevent paradoxical embolisms and early shunt closure (Figure).
A: advancement of the sheath until the left atrium via the fossa ovalis. B, C, and D: images from angiography (B), bench testing (C), and 3D echocardiography (D) of the opening of the left part of the device in the left atrium. E, F, and G: images from bench testing (E), angiography (F), and color Doppler echocardiography (G) of the unfolded V-Wave.
The first patient was described in Canada6 and the technology has recently been introduced in Europe, where the first 2 implants were successfully performed with the approval of the Agencia Española de Medicamentos y Productos Sanitarios (Spanish Agency for Medicines and Medical Devices). The selection criteria for this first-in-man study are summarized in the Table. Detailed clinical (Kansas City Cardiomyopathy Questionnaire [KCCQ]) and functional (6-minute walk test) tests were performed, as well as measurement of the aminoterminal fraction of brain natriuretic peptide (NT-proBNP) and right heart catheterization.
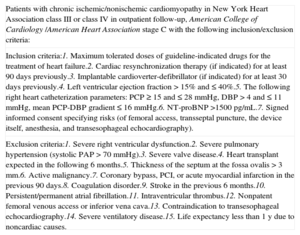
Summary of the Inclusion and Exclusion Criteria for Candidates of the V-Wave Device in this First-in-man Study
| Patients with chronic ischemic/nonischemic cardiomyopathy in New York Heart Association class III or class IV in outpatient follow-up, American College of Cardiology /American Heart Association stage C with the following inclusion/exclusion criteria: |
| Inclusion criteria:1. Maximum tolerated doses of guideline-indicated drugs for the treatment of heart failure.2. Cardiac resynchronization therapy (if indicated) for at least 90 days previously.3. Implantable cardioverter-defibrillator (if indicated) for at least 30 days previously.4. Left ventricular ejection fraction > 15% and ≤ 40%.5. The following right heart catheterization parameters: PCP ≥ 15 and ≤ 28mmHg, DBP > 4 and ≤ 11mmHg, mean PCP-DBP gradient ≤ 16mmHg.6. NT-proBNP >1500 pg/mL.7. Signed informed consent specifying risks (of femoral access, transseptal puncture, the device itself, anesthesia, and transesophageal echocardiography). |
| Exclusion criteria:1. Severe right ventricular dysfunction.2. Severe pulmonary hypertension (systolic PAP > 70 mmHg).3. Severe valve disease.4. Heart transplant expected in the following 6 months.5. Thickness of the septum at the fossa ovalis > 3mm.6. Active malignancy.7. Coronary bypass, PCI, or acute myocardial infarction in the previous 90 days.8. Coagulation disorder.9. Stroke in the previous 6 months.10. Persistent/permanent atrial fibrillation.11. Intraventricular thrombus.12. Nonpatent femoral venous access or inferior vena cava.13. Contraindication to transesophageal echocardiography.14. Severe ventilatory disease.15. Life expectancy less than 1 y due to noncardiac causes. |
DBP, diastolic blood pressure; NT-proBNP, aminoterminal fraction of brain natriuretic peptide; PAP, pulmonary artery pressure; PCI, percutaneous coronary intervention; PCP, pulmonary capillary pressure.
The first patient was a 73-year-old man with partially revascularized chronic ischemic heart disease and a cardiac resynchronization therapy device. He had severe left ventricular dysfunction (24%), moderate mitral regurgitation (II/IV), and acceptable right ventricular function (tricuspid annular plane systolic excursion [TAPSE] of 14mm). His 6-minute walk test distance was 253 meters and his KCCQ score was 35.42, and the right heart catheter showed cardiac output of 3.5 L/min, pulmonary capillary pressure of 26mmHg, and right atrial pressure of 10mmHg. The patient's NT-proBNP level was 3341 pg/mL.
The second patient was a 70-year-old man with surgically and percutaneously revascularized ischemic dilated cardiomyopathy and a cardioverter-defibrillator. He had left ventricular dysfunction (28%), moderate mitral regurgitation (II/IV), and TAPSE of 19mm. His 6-minute walk test distance was 236 meters and his KCCQ score was 42.45, and the right heart catheter showed cardiac output of 4.8 L/min, pulmonary capillary pressure of 16mmHg, and right atrial pressure of 5mmHg. The patient's NT-proBNP level was 1526 pg/mL.
Despite receiving optimal medical therapy and high-dose diuretics (120mg and 80mg, respectively), both patients showed New York Heart Association (NYHA) functional class III dyspnea and orthopnea. Approval was obtained from a committee of heart failure experts and, once an informed consent form had been signed, we decided to implant the V-Wave device. The procedure was performed under general anesthesia to improve the tolerability of transesophageal echocardiography. The right atrium was accessed via a femoral vein approach, a transseptal puncture was made at the level of the fossa ovalis, and a 14-Fr sheath was inserted into the left atrium (Figure A-D). The device was attached to a delivery catheter and advanced through the sheath until its first portion opened in the middle part of the left atrium. Once correct apposition of the device to the septum was confirmed, the device was released from the delivery catheter. After a slight pull of the sheath, the second part of the device opened in the right atrium and the correct fixation of the device in the interatrial septum was confirmed (Figure E-G; see the video of the supplementary material). Following implantation, the left-right shunt was immediately visualized in both patients via transesophageal echocardiography (Figure G). Both patients were discharged 24hours after the procedure with no complications and with instructions for 3-month oral anticoagulation therapy, during which time no other therapeutic modifications could be performed according to the protocol.
At a 3-month follow-up, the V-Wave device was patent and both patients were in NYHA functional class II, without orthopnea and with KCCQ scores of 62.5 and 63.54, respectively. Improvements were also evident in objective parameters, such as increases of 27.3% and 19.0% in the 6-minute walk test distance, NT-proBNP values of 2663 pg/mL and 1129 pg/mL, cardiac output of 4.6 L/min, and pulmonary capillary pressure of 23mmHg for the first patient (Qp:Qs = 1.3) and 5.2 L/min and 12mmHg for the second (Qp:Qs = 1.2). Nonetheless, the promising results of this new therapeutic approach require long-term validation.
CONFLICTS OF INTERESTSJ. Rodés-Cabau is a consultant for V-Wave Ltd.
We thank Drs. Gimeno, López, Arnold, de la Fuente, Urueña, Pastor, and Mota for their vital contributions to the success of this project.