In Spain, the prevalence of heart failure (HF) exceeds 15% among the elderly.1 Managing HF consumes 1% to 2% of the healthcare budget and hospitalizations consume two-thirds of this expenditure.2 Diuretics are the main treatment and furosemide is the most commonly used drug. Its action may be decreased by multiple factors, leading to the need for intravenous infusion3 and often involving hospitalization. Experience in the use of subcutaneous (s.c.) furosemide is scarce and the systems most frequently used are dependent on health care personnel.4 Elastomeric infusion pumps are nonelectric, disposable, continuous-flow pumps that are widely used in antimicrobial therapy, analgesia, and cancer treatment.5 They are little used in HF, but could be an alternative diuretic treatment for patients with decompensated HF. We describe the response to treatment of a series of ambulatory HF patients with indications for parenteral diuretic therapy treated with s.c. furosemide using elastomeric pumps.
Between December 2010 and December 2011, s.c. furosemide was administered in the Heart Failure Unit (University Hospital, Valladolid, Spain) to resolve 41 episodes in 24 patients with decompensated Heart Failure. We included 65 clinical and laboratory variables. Patients who did not meet the criteria for parenteral administration were excluded.
Continuous s.c. furosemide was administered by elastomeric pump in an outpatient setting. Treatment was given for 4 or 5 days (96 mL at 1 mL/h or 240 mL at 2 mL/h) depending on pump volume and preset flow rate. The pumps were connected to a catheter (Abbocath 20-22G®) implanted subcutaneously in chest or abdominal tissue (Figure A). The catheter was maintained indefinitely in the absence of complications. The daily dose was calculated at the discretion of the prescribing physician and administered in dextrose 5%. Patients were monitored in the HF Unit every 5 to 7 days. Blood was collected at baseline and at the end of treatment. The effectiveness of treatment was assessed by weight loss. “End of treatment” was achieved when functional class improved or dry weight was maintained without parenteral diuretic therapy.
A, infusion pump, components, and subcutaneous implantation technique (1, infusion pump; 2, elastomeric reservoir with furosemide in dextrose 5%; 3, flow controller; 4, extension; 5, plastic catheter implanted subcutaneously in chest tissue; 6, transparent patch). B, variations (?) in weight and serum creatinine, potassium, and sodium at the beginning and end of treatment with a confidence interval of 95% (95%CI). Significant weight loss was observed.
A descriptive analysis was conducted and the normality of distribution of the variables tested using the Shapiro-Wilks test. The Student t test was used for normal variables and the Wilcoxon test for non-normal variables using the SPSS® V18 software package (SPSS Inc.).
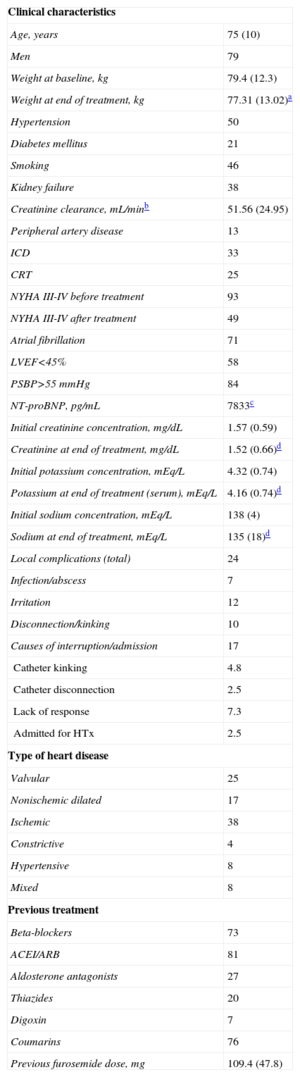
Mean age was 75 (10) years, 79% were male, and 93% were in an advanced functional class at baseline. The Table shows the characteristics of the population.
Clinical and Laboratory Characteristics of the Patients and Treatments Administered
| Clinical characteristics | |
| Age, years | 75 (10) |
| Men | 79 |
| Weight at baseline, kg | 79.4 (12.3) |
| Weight at end of treatment, kg | 77.31 (13.02)a |
| Hypertension | 50 |
| Diabetes mellitus | 21 |
| Smoking | 46 |
| Kidney failure | 38 |
| Creatinine clearance, mL/minb | 51.56 (24.95) |
| Peripheral artery disease | 13 |
| ICD | 33 |
| CRT | 25 |
| NYHA III-IV before treatment | 93 |
| NYHA III-IV after treatment | 49 |
| Atrial fibrillation | 71 |
| LVEF<45% | 58 |
| PSBP>55 mmHg | 84 |
| NT-proBNP, pg/mL | 7833c |
| Initial creatinine concentration, mg/dL | 1.57 (0.59) |
| Creatinine at end of treatment, mg/dL | 1.52 (0.66)d |
| Initial potassium concentration, mEq/L | 4.32 (0.74) |
| Potassium at end of treatment (serum), mEq/L | 4.16 (0.74)d |
| Initial sodium concentration, mEq/L | 138 (4) |
| Sodium at end of treatment, mEq/L | 135 (18)d |
| Local complications (total) | 24 |
| Infection/abscess | 7 |
| Irritation | 12 |
| Disconnection/kinking | 10 |
| Causes of interruption/admission | 17 |
| Catheter kinking | 4.8 |
| Catheter disconnection | 2.5 |
| Lack of response | 7.3 |
| Admitted for HTx | 2.5 |
| Type of heart disease | |
| Valvular | 25 |
| Nonischemic dilated | 17 |
| Ischemic | 38 |
| Constrictive | 4 |
| Hypertensive | 8 |
| Mixed | 8 |
| Previous treatment | |
| Beta-blockers | 73 |
| ACEI/ARB | 81 |
| Aldosterone antagonists | 27 |
| Thiazides | 20 |
| Digoxin | 7 |
| Coumarins | 76 |
| Previous furosemide dose, mg | 109.4 (47.8) |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; CRT, cardiac resynchronization therapy; HTx, heart transplantation; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association functional class; PSBP, pulmonary systolic blood pressure.
Unless otherwise indicated, values are percentages.
The average time of treatment was 9 (4) days. In addition to s.c. furosemide, in 39% of the episodes oral furosemide was maintained to avoid changing chronic treatment. The average total dose during therapy was 179 (48) mg/d and the mean subcutaneous infusion dose was 146 (40) mg/d. The typical initial infusion dose was 120 mg/d (56%). There was significant weight loss, but no significant changes in serum creatinine, potassium, and sodium concentrations (Table; Figure, B). There was one case of hypokalemia (serum potassium level <3 mEq/L). Functional class improved in 61% of patients, did not change in 36%, and worsened in 3%. There were no treatment-related deaths. Hospital admission or interruption of treatment was required in 17%. The Table shows their cause as well as local adverse effects.
We present the largest series of patients with decompensated HF treated with continuous s.c. furosemide administered by elastomeric infusion pumps. This route is usually restricted to terminally ill patients at home.6 Elastomeric infusion pumps provide safe comprehensive outpatient treatment without the need for daily monitoring, thus allowing scheduled examinations. It is well tolerated, effective in preventing hospital admissions, and decreases costs due to the low price of the devices (about 30 euros per device).
The clinical profile of this sample of elderly patients, who were in an advanced functional class and with serious comorbidity, did not hinder the response to treatment; significant weight loss prevented a possible hospital admission in 83% of the sample.
The administration of s.c. furosemide by electric infusion pump has been shown to be effective in patients who are terminally ill or have decompensated HF,4 as assessed by weight loss and improvement in symptoms, although safety data remain unknown. Unlike elastomeric pumps, electronic pumps present some disadvantages such as limited patient autonomy, increased noise levels, the need for maintenance, and dependence on health care personnel.
The use of s.c. furosemide in HF patients could solve some of the typical problems that arise when treating these patients: venipuncture failure, intravenous catheter infection, failure to adhere to treatment, repeat admissions, and the inability to wean patients from intravenous diuretics. This implantation route is accessible in 100% of patients, presents no technical difficulties, and is not painful; the device is simple and comfortable to transport, facilitates toileting, and allows patient mobility.
Local adverse effects are common with this route,6 but are of little clinical importance. In our series, there was only 1 case of s.c. abscess that needed drainage. These reactions can be avoided by the use of plastic catheters, aseptic procedures, and a good implantation technique.
Although this study is limited by the small number of episodes, it demonstrates the efficacy and safety of the use of continuous s.c. infusion of furosemide to treat ambulatory patients with decompensated HF.
FUNDINGThis study was funded in part by the Red de Centros Cardiovasculares (RECAVA), which is funded by the Instituto de Salud Carlos III.
.