Although the definition of nonvalvular atrial fibrillation (NVAF) varies,1,2 it generally does not exclude patients with structural heart disease (SHD), such as certain valve diseases. However, there is limited information on the frequency of this association in Spain. The objective of this article was to report the prevalence and clinical profile of patients with SHD and well as the prevalence of heart failure in a broad Spanish nationwide sample of patients with NVAF.
Data from the FANTASIIA registry3 were used. This registry included 2178 outpatients with NVAF who were receiving anticoagulation (according to protocol, the ratio of vitamin K antagonists to direct anticoagulants was 4:1). We excluded individuals younger than 18 years, those with prosthetic cardiac devices, those with any grade of mitral stenosis, and those with moderate or severe mitral regurgitation. Participants were enrolled consecutively between June 1, 2013, and October 15, 2014, in 50 Spanish centers selected by the investigators to ensure representation from throughout the country, with the primary objective of assessing the effectiveness of anticoagulation in patients with NVAF by type and quality of treatment. The diagnoses of SHD were taken from the medical records and included the following: coronary artery disease, hypertensive heart disease, dilated cardiomyopathy, hypertrophic cardiomyopathy, significant valve disease (aortic valve, tricuspid valve, or pulmonary valve disease of at least moderate intensity), and other heart diseases. Patients with coronary artery diseases and other concurrent heart diseases were classified as having coronary artery disease. The presence of heart failure was recorded independently. Overall, 47.15% of the sample had SHD (Table 1). The most frequent type of SHD was coronary artery disease (18.14%), followed by hypertensive heart diseases (11.43%), and dilated cardiomyopathy (6.01%). Hypertrophic cardiomyopathy was reported in 2.06% and significant valve diseases in 1.79%. Only 34 patients (1.56%) had isolated NVAF (age < 65 years, with no heart disease or embolic risk factor). Among patients with SHD, there were fewer women and there was a higher rate of cardiovascular risk factors, comorbidities, heart failure, permanent atrial fibrillation, and severe symptoms, and greater embolic and hemorrhagic risk. These patients also had worse left ventricular ejection fractions and renal function, as well as lower hemoglobin levels. Most of the drug classes were more frequently prescribed in patients with SHD, except for angiotensin receptor blockers (prescribed with a similar frequency) and antiarrhythmics and direct anticoagulants (prescribed less often). Overall, 27.23% of the patients had heart failure, with differential characteristics with respect to the sample, similar to patients with SHD, with a few exceptions (Table 2). Studies in Spain have reported a prevalence of coronary artery disease of between 10% and 20% in anticoagulated patients with NVAF,4–6 a similar prevalence to that reported in our study. The CALIFA registry is the only one of these studies to report frequencies of hypertensive heart failure (15.7%) and valve disease (4%) in Spain.4 These frequencies are similar to those reported in our registry (11.4% and 2%, respectively). It is possible that exclusion of patients with moderate or severe mitral regurgitation could partly explain this low frequency of heart disease. In the case of heart failure, previous studies have reported frequencies between 22% and 24%,4–6 which are similar to those observed in this series. A limitation of the present study is that several design features (anticoagulation in the 6 months prior to inclusion, exclusion of hospitalized patients, the willingness of the physicians involved in the registry, etc) could have resulted in a biased sample, and so extrapolation of our results to the overall population with NVAF should be made with caution. Furthermore, classification of heart disease was performed using medical records, which, although a true reflection of everyday clinical practice, may have heterogeneous application of diagnostic criteria. Nevertheless, our results, obtained in a large Spanish sample of consecutive patients with NVAF, suggest that almost half have SHD and more than quarter have heart failure. These patients had a different clinical profile to the other patients with NVAF and they received direct anticoagulants less frequently.
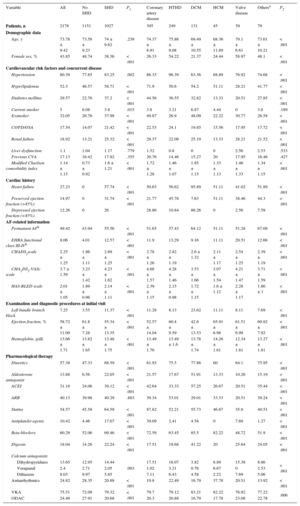
General Characteristics of the Patients Included in the Study by Presence and Type of Structural Heart Disease
| Variable | All | No SHD | SHD | P1 | Coronary artery disease | HTHD | DCM | HCM | Valve disease | Othersa | P2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | 2178 | 1151 | 1027 | 395 | 249 | 131 | 45 | 39 | 79 | ||
| Demographic data | |||||||||||
| Age, y | 73.78 ± 9.42 | 73.58 ± 9.23 | 74 ± 9.62 | .239 | 74.37 ± 8.91 | 75.86 ± 9.08 | 69.49 ± 10.55 | 68.36 ± 11.89 | 79.1 ± 6.61 | 73.61 ± 10.21 | < .001 |
| Female sex, % | 43.85 | 48.74 | 38.36 | < .001 | 26.33 | 54.22 | 21.37 | 24.44 | 58.97 | 48.1 | < .001 |
| Cardiovascular risk factors and concurrent disease | |||||||||||
| Hypertension | 80.39 | 77.85 | 83.25 | .002 | 86.33 | 96.39 | 63.36 | 68.89 | 76.92 | 74.68 | < .001 |
| Hyperlipidemia | 52.3 | 46.57 | 58.71 | < .001 | 71.9 | 50.6 | 54.2 | 51.11 | 28.21 | 41.77 | < .001 |
| Diabetes mellitus | 29.57 | 22.76 | 37.2 | < .001 | 44.56 | 36.55 | 32.82 | 13.33 | 20.51 | 27.85 | < .001 |
| Current smoker | 5 | 6.08 | 3.8 | .015 | 3.8 | 3.21 | 6.87 | 4.44 | 0 | 3.8 | .189 |
| Exsmoker | 32.05 | 26.76 | 37.98 | < .001 | 49.87 | 26.9 | 48.09 | 22.22 | 30.77 | 26.58 | < .001 |
| COPD/OSA | 17.54 | 14.07 | 21.42 | < .001 | 22.53 | 24.1 | 19.85 | 15.56 | 17.95 | 17.72 | < .001 |
| Renal failure | 18.92 | 13.21 | 25.32 | < .001 | 29.37 | 22.09 | 25.19 | 13.33 | 28.21 | 21.52 | < .001 |
| Liver dysfunction | 1.1 | 1.04 | 1.17 | .779 | 1.52 | 0.8 | 0 | 0 | 2.56 | 2.53 | .533 |
| Previous CVA | 17.13 | 16.42 | 17.92 | .355 | 20.76 | 14.46 | 15.27 | 20 | 17.95 | 16.46 | .427 |
| Modified Charlson comorbidity index | 1.14 ± 1.15 | 0.73 ± 0.92 | 1.6 ± 1.21 | < .001 | 1.72 ± 1.28 | 1.46 ± 1.07 | 1.85 ± 1.15 | 1.33 ± 1.13 | 1.46 ± 1.33 | 1.34 ± 1.15 | < .001 |
| Cardiac history | |||||||||||
| Heart failure | 27.23 | 0 | 57.74 | < .001 | 50.63 | 56.62 | 95.89 | 51.11 | 41.02 | 51.89 | < .001 |
| Preserved ejection fraction (>45%) | 14.97 | 0 | 31.74 | < .001 | 21.77 | 45.78 | 7.63 | 51.11 | 38.46 | 44.3 | < .001 |
| Depressed ejection fraction (<45%) | 12.26 | 0 | 26 | 28.86 | 10.84 | 86.26 | 0 | 2.56 | 7.59 | ||
| AF-related information | |||||||||||
| Permanent AFb | 49.42 | 43.94 | 55.56 | < .001 | 51.65 | 57.43 | 64.12 | 51.11 | 51.28 | 67.09 | < .001 |
| EHRA functional class III-IVb | 8.06 | 4.01 | 12.57 | < .001 | 11.9 | 13.29 | 9.16 | 11.11 | 20.51 | 12.66 | < .001 |
| CHADS2scale | 2.25 ± 1.25 | 1.86 ± 1.11 | 2.69 ± 1.25 | < .001 | 2.78 ± 1.26 | 2.82 ± 1.19 | 2.6 ± 1.32 | 2.11 ± 1.17 | 2.54 ± 1.25 | 2.39 ± 1.19 | < .001 |
| CHA2DS2-VASc scale | 3.7 ± 1.59 | 3.23 ± 1.42 | 4.23 ± 1.62 | < .001 | 4.68 ± 1.57 | 4.28 ± 1.46 | 3.53 ± 1.66 | 3.07 ± 1.54 | 4.21 ± 1.47 | 3.71 ± 1.59 | < .001 |
| HAS-BLED scale | 2.01 ± 1.05 | 1.89 ± 0.96 | 2.14 ± 1.11 | < .001 | 2.39 ± 1.15 | 2.15 ± 0.98 | 1.72 ± 1.15 | 1.6 ± 1.12 | 2.28 ± 1.17 | 1.86 ± 1 | < .001 |
| Examination and diagnostic procedures at initial visit | |||||||||||
| Left bundle branch block | 7.25 | 3.55 | 11.37 | < .001 | 11.28 | 8.13 | 23.62 | 11.11 | 8.11 | 7.69 | < .001 |
| Ejection fraction, % | 58.72 ± 11.09 | 61.8 ± 7.28 | 55.34 ± 13.35 | < .001 | 52.57 ± 14.04 | 60.4 ± 9.59 | 42.6 ± 13.53 | 65.91 ± 6.98 | 61.51 ± 6.99 | 60.92 ± 7.92 | < .001 |
| Hemoglobin, g/dL | 13.66 ± 1.71 | 13.82 ± 1.65 | 13.48 ± 1.75 | < .001 | 13.49 ± 1.76 | 13.49 ± 1.6 | 13.78 ± 1.74 | 14.26 ± 1.61 | 12.34 ± 1.81 | 13.27 ± 1.81 | < .001 |
| Pharmacological therapy | |||||||||||
| Diuretics | 57.38 | 47.33 | 68.59 | < .001 | 61.93 | 75.5 | 77.86 | 60 | 64.1 | 75.95 | < .001 |
| Aldosterone antagonist | 13.88 | 6.56 | 22.05 | < .001 | 21.57 | 17.67 | 51.91 | 13.33 | 10.26 | 15.19 | < .001 |
| ACEI | 31.18 | 24.06 | 39.12 | < .001 | 42.64 | 33.33 | 57.25 | 26.67 | 20.51 | 35.44 | < .001 |
| ARB | 40.13 | 39.98 | 40.29 | .883 | 39.34 | 53.01 | 29.01 | 33.33 | 20.51 | 39.24 | < .001 |
| Statins | 54.57 | 45.58 | 64.59 | < .001 | 87.82 | 52.21 | 55.73 | 46.67 | 35.9 | 40.51 | < .001 |
| Antiplatelet agents | 10.42 | 4.46 | 17.07 | < .001 | 39.09 | 2.41 | 4.58 | 0 | 7.69 | 1.27 | < .001 |
| Beta-blockers | 60.29 | 52.06 | 69.46 | < .001 | 72.59 | 63.45 | 85.5 | 82.22 | 48.72 | 51.9 | < .001 |
| Digoxin | 18.04 | 14.26 | 22.24 | < .001 | 17.51 | 19.68 | 41.22 | 20 | 25.64 | 24.05 | < .001 |
| Calcium antagonists | |||||||||||
| Dihydropyridines | 13.65 | 12.95 | 14.44 | .003 | 17.51 | 18.07 | 3.82 | 8.89 | 15.38 | 8.86 | < .001 |
| Verapamil | 2.4 | 2.71 | 2.05 | 1.02 | 3.21 | 0.76 | 6.67 | 0 | 2.53 | ||
| Diltiazem | 8.03 | 9.97 | 5.85 | 7.11 | 6.43 | 4.58 | 2.22 | 7.69 | 5.06 | ||
| Antiarrhythmics | 24.82 | 28.35 | 20.88 | < .001 | 19.8 | 22.49 | 16.79 | 37.78 | 20.51 | 13.92 | < .001 |
| VKA | 75.51 | 72.09 | 79.32 | < .001 | 79.7 | 79.12 | 83.21 | 82.22 | 76.92 | 77.22 | .006 |
| ODAC | 24.49 | 27.91 | 20.68 | 20.3 | 20.88 | 16.79 | 17.78 | 23.08 | 22.78 | ||
ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; COPD/OSA, chronic obstructive pulmonary diseases/obstructive sleep apnea syndrome; CVA, cerebrovascular accident; DCM, dilated cardiomyopathy; EHRA, European Heart Rhythm Association; HCM, hypertrophic cardiomyopathy; HTHD, hypertensive heart disease; ODAC, oral direct anticoagulants; SHD, structural heart disease; VKA, vitamin K antagonist. P1, comparison between patients with SHD and without SHD (Mann-Whitney test for continuous variables and chi-square test for categorical variables); P2, comparison between patients without SHD, coronary artery diseases, HTHD, DCM, HCM, valve diseases, and others (Kruskal-Wallis test for continuous variables and chi-square test for categorical variables).
According to the European Society of Cardiology.1
c Flecainide, propafenone, amiodarone, dronedarone, or sotalol.
Quantitative data expressed as mean ± standard deviation and qualitative data as percentages.
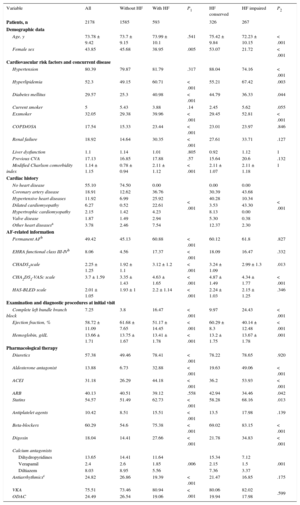
General Characteristics of the Patients Included in the Study by Presence and Type of Heart Failure
| Variable | All | Without HF | With HF | P1 | HF conserved | HF impaired | P2 |
|---|---|---|---|---|---|---|---|
| Patients, n | 2178 | 1585 | 593 | 326 | 267 | ||
| Demographic data | |||||||
| Age, y | 73.78 ± 9.42 | 73.7 ± 9.15 | 73.99 ± 10.1 | .541 | 75.42 ± 9.84 | 72.23 ± 10.15 | < .001 |
| Female sex | 43.85 | 45.68 | 38.95 | .005 | 53.07 | 21.72 | < .001 |
| Cardiovascular risk factors and concurrent disease | |||||||
| Hypertension | 80.39 | 79.87 | 81.79 | .317 | 88.04 | 74.16 | < .001 |
| Hyperlipidemia | 52.3 | 49.15 | 60.71 | < .001 | 55.21 | 67.42 | .003 |
| Diabetes mellitus | 29.57 | 25.3 | 40.98 | < .001 | 44.79 | 36.33 | .044 |
| Current smoker | 5 | 5.43 | 3.88 | .14 | 2.45 | 5.62 | .055 |
| Exsmoker | 32.05 | 29.38 | 39.96 | < .001 | 29.45 | 52.81 | < .001 |
| COPD/OSA | 17.54 | 15.33 | 23.44 | < .001 | 23.01 | 23.97 | .846 |
| Renal failure | 18.92 | 14.64 | 30.35 | < .001 | 27.61 | 33.71 | .127 |
| Liver dysfunction | 1.1 | 1.14 | 1.01 | .805 | 0.92 | 1.12 | 1 |
| Previous CVA | 17.13 | 16.85 | 17.88 | .57 | 15.64 | 20.6 | .132 |
| Modified Charlson comorbidity index | 1.14 ± 1.15 | 0.78 ± 0.94 | 2.11 ± 1.12 | < .001 | 2.11 ± 1.07 | 2.11 ± 1.18 | 1 |
| Cardiac history | |||||||
| No heart disease | 55.10 | 74.50 | 0.00 | < .001 | 0.00 | 0.00 | < .001 |
| Coronary artery disease | 18.91 | 12.62 | 36.76 | 30.39 | 43.68 | ||
| Hypertensive heart diseases | 11.92 | 6.99 | 25.92 | 40.28 | 10.34 | ||
| Dilated cardiomyopathy | 6.27 | 0.52 | 22.61 | 3.53 | 43.30 | ||
| Hypertrophic cardiomyopathy | 2.15 | 1.42 | 4.23 | 8.13 | 0.00 | ||
| Valve disease | 1.87 | 1.49 | 2.94 | 5.30 | 0.38 | ||
| Other heart diseasesa | 3.78 | 2.46 | 7.54 | 12.37 | 2.30 | ||
| AF-related information | |||||||
| Permanent AFb | 49.42 | 45.13 | 60.88 | < .001 | 60.12 | 61.8 | .827 |
| EHRA functional class III-IVb | 8.06 | 4.56 | 17.37 | < .001 | 18.09 | 16.47 | .332 |
| CHADS2scale | 2.25 ± 1.25 | 1.92 ± 1.1 | 3.12 ± 1.2 | < .001 | 3.24 ± 1.09 | 2.99 ± 1.3 | .013 |
| CHA2DS2-VASc scale | 3.7 ± 1.59 | 3.35 ± 1.43 | 4.63 ± 1.65 | < .001 | 4.87 ± 1.49 | 4.34 ± 1.77 | < .001 |
| HAS-BLED scale | 2.01 ± 1.05 | 1.93 ± 1 | 2.2 ± 1.14 | < .001 | 2.24 ± 1.03 | 2.15 ± 1.25 | .346 |
| Examination and diagnostic procedures at initial visit | |||||||
| Complete left bundle branch block | 7.25 | 3.8 | 16.47 | < .001 | 9.97 | 24.43 | < .001 |
| Ejection fraction, % | 58.72 ± 11.09 | 61.68 ± 7.65 | 51.17 ± 14.45 | < .001 | 60.29 ± 8.3 | 40.14 ± 12.48 | < .001 |
| Hemoglobin, g/dL | 13.66 ± 1.71 | 13.75 ± 1.67 | 13.41 ± 1.78 | < .001 | 13.2 ± 1.75 | 13.67 ± 1.78 | .001 |
| Pharmacological therapy | |||||||
| Diuretics | 57.38 | 49.46 | 78.41 | < .001 | 78.22 | 78.65 | .920 |
| Aldosterone antagonist | 13.88 | 6.73 | 32.88 | < .001 | 19.63 | 49.06 | < .001 |
| ACEI | 31.18 | 26.29 | 44.18 | < .001 | 36.2 | 53.93 | < .001 |
| ARB | 40.13 | 40.51 | 39.12 | .558 | 42.94 | 34.46 | .042 |
| Statins | 54.57 | 51.49 | 62.73 | < .001 | 58.28 | 68.16 | .013 |
| Antiplatelet agents | 10.42 | 8.51 | 15.51 | < .001 | 13.5 | 17.98 | .139 |
| Beta-blockers | 60.29 | 54.6 | 75.38 | < .001 | 69.02 | 83.15 | < .001 |
| Digoxin | 18.04 | 14.41 | 27.66 | < .001 | 21.78 | 34.83 | < .001 |
| Calcium antagonists | |||||||
| Dihydropyridines | 13.65 | 14.41 | 11.64 | .006 | 15.34 | 7.12 | .001 |
| Verapamil | 2.4 | 2.6 | 1.85 | 2.15 | 1.5 | ||
| Diltiazem | 8.03 | 8.95 | 5.56 | 7.36 | 3.37 | ||
| Antiarrhythmicsc | 24.82 | 26.86 | 19.39 | < .001 | 21.47 | 16.85 | .175 |
| VKA | 75.51 | 73.46 | 80.94 | < .001 | 80.06 | 82.02 | .599 |
| ODAC | 24.49 | 26.54 | 19.06 | 19.94 | 17.98 | ||
ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; COPD/OSA, chronic obstructive pulmonary diseases/obstructive sleep apnea syndrome; CVA, cerebrovascular accident; EHRA, European Heart Rhythm Association; HF, heart failure; ODAC, oral direct anticoagulants; SHD, structural heart disease; VKA, vitamin K antagonist. P1, comparison between patients with HF and without HF (Mann-Whitney test for continuous variables and chi-square test for categorical variables); P2, comparison between patients with HF and conserved systolic function and patients with HF and depressed systolic function (Kruskal-Wallis test for continuous variables and chi-square test for categorical variables).
This study was funded with a research grant from Pfizer/Bristol-Myers-Squibb.