Hybrid procedures, which involve banding of both pulmonary arteries and percutaneous ductal stenting, eliminate the need for extracorporeal circulation in high-risk newborns with congenital heart disease. They have mainly been described in hypoplastic left heart syndrome,2 but more recently, they have been used to treat different forms of left-sided obstructive heart lesions, such as interrupted aortic arch.3
We present the case of a newborn with type B interrupted aortic arch and a large perimembranous ventricular septal defect. The perinatal history was remarkable for intrauterine growth restriction type 1, a gestational age of 37 weeks with a birth weight of 2059g, and anal atresia.
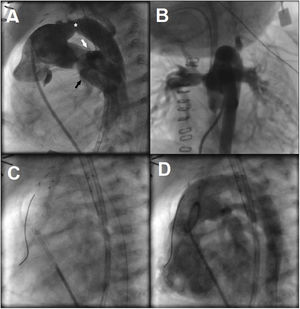
Considering the patient's low birth weight, large ventricular septal defect, and digestive malformation, it was decided to perform a hybrid procedure. At the age of 28 days and weighing 2500g, the patient underwent bilateral pulmonary artery banding. The intervention was successful and resulted in a maximum pressure gradient of 70mmHg in both arteries. At 34 days of age, he was taken to the catheterization laboratory for ductal stenting. Pulmonary angiography showed a ductus arteriosus with a length of 18mm and a diameter of 5mm at its narrowest point and 7mm at its ends (figure 1A,B and video 1 of the supplementary data). An 8×18-mm self-expanding Sinus-SuperFlex-DS (SSF) stent (OptiMed, Germany) was inserted in the ductus arteriosus through an arterial access (figure 1C,D and video 2 of the supplementary data). The procedure was successful and uneventful. Prostaglandin infusion was discontinued and the patient was started on antiplatelet therapy with aspirin.
A: lateral angiographic projection of the pulmonary trunk. Note the stenosis in the middle third of the ductus arteriosus (asterisk), which is connected to the distal portion of the interrupted aortic arch in the upper part and the left pulmonary artery (white arrow) and right pulmonary artery (black arrow) in the lower part. B: anterior-posterior angiographic projection of the pulmonary trunk. C: arterial placement of Sinus-SuperFlex-DS stent in the ductus arteriosus. D: pulmonary angiogram after the procedure.
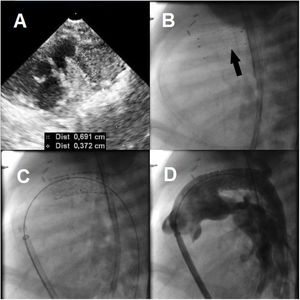
The follow-up echocardiogram performed 24hours after the procedure showed an in-stent restenosis of 3.7mm in the middle third of the ductus arteriosus and a maximum pressure gradient of 70mmHg (4.1m/s) in the right-to-left shunt (figure 2A). Progressive oliguria was also evident at this time. Prostaglandin infusion was restarted and resulted in a reduction in the pressure gradient to 15mmHg (1.9m/s) and partial re-expansion of the stent. Imaging in the catheterization laboratory confirmed that the stent had collapsed in the middle third of the ductus arteriosus and was unstable (figure 2B and video 3 of the supplementary data). A Formula stent (8×24mm) was successfully placed through the original stent (figure 2C,D), and in the following hours, the patient achieved hemodynamic stability, enabling removal of respiratory support. He developed sepsis, however, and died 10 days later.
Interrupted aortic arch is a rare, duct-dependent, congenital heart defect. It could be considered the most extreme presentation of coarctation of the aorta, and a ventricular septal defect may also be present. Although direct end-to-end anastomosis is the treatment of choice, comorbid conditions or other lesions increase the risk of surgical complications and morbidity and mortality.3 Hybrid procedures provide a satisfactory temporary solution in such cases.
Balloon-expandable stents were used in early hybrid procedures,1 but self-expanding stents specifically designed for neonatal ductal stenting appeared in later years. One example is the SSF stent, which, while designed for the hybrid treatment of hypoplastic left heart syndrome, has been used to treat numerous duct-dependent congenital heart lesions. Although SSF stents are made of nitinol, they might have a lower radial force than balloon-expandable stents.4 There have been reports of ductal SSF stent collapse, mostly in patients such as ours, who have some degree of ductus arteriosus stenosis prior to stenting. Betrián-Blanco et al.5 published the case of a newborn with hypoplastic left heart syndrome who underwent 2-stent telescoping and experienced collapse of the first stent within 48hours. More recently, Hribernik et al.6 presented 5 cases of SSF stents that collapsed within weeks of placement.
Considering the above, we believe that ductal stenosis should be taken into account when choosing a stent. In patients with stenosis, balloon-expandable stents might provide better radial force than self-expanding stents.4 Self-expanding SSF stent collapse has been described. Close clinical and ultrasound monitoring is recommended during the first hours and days after stenting.
Written informed consent was obtained from the patient's legal representative for the publication of this case and accompanying images.
FundingNo funding.
Authors’ ContributionsP. Agudo-Montore, I. Guillén-Rodríguez, and F. Coserria-Sánchez reviewed the literature, selected the images and videos, and drafted this manuscript. B. Manso-García and A. González-Calle revised and edited the manuscript. All the authors were involved in the patient's treatment and revised and approved the final manuscript.
Conflicts of InterestNo conflicts of interest.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.05.014