In recent years, high blood concentrations of galectin-3 (Gal-3) have been associated with a worse clinical outcome in patients with heart failure (HF).1 In patients hospitalized due to acute HF, high concentrations are associated with an increased risk of death and rehospitalization.2 However, it has not been established whether this phenotype is modifiable in the short-term; therefore, the aim of this study was to evaluate the kinetics of Gal-3 in the first 30 days following an episode of acute HF.
The study included 109 patients admitted due to acute HF (60% were male; mean age, 71±11 years; left ventricular ejection fraction, 41±15%). Serial blood samples were taken at 3 distinct time points: a) on arrival at the emergency department (first sample; n=109); b) on the day of discharge (median stay, 7 days; n=109), and c) 30 days after discharge (n=98). The Gal-3 concentrations were measured with the automated immunoassay system VIDAS (miniVidas, bioMérieux). A cutoff value of 17.8 ng/mL, the 90th percentile of normal, was taken to be a high concentration, as per the data sheet and the approved use for risk stratification.3
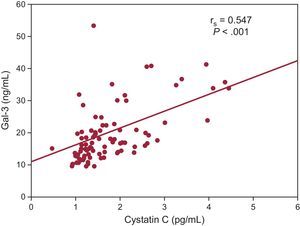
The median Gal-3 concentration on arrival at the emergency department was 17.2 ng/mL [interquartile range, 13.9-22.9 ng/mL]. When we used the cutoff value of 17.8 ng/mL, those patients with a higher concentration (n=50) were older (76 [68-81] vs 71 [60-78] years; P=.027), a higher proportion had previously been admitted for HF (58% vs 34%; P=.013), and more of them required intravenous dopamine (20% vs 5%; P=.020). Gal-3 concentration correlated significantly with worse renal function parameters: urea (rs=0.50; P<.001), creatinine (rs=0.423; P<.001), estimated glomerular filtration rate (rs=–0.53; P<.001), and cystatin C (rs=0.55; P<.001); and with higher concentrations of N-terminal probrain natriuretic peptide (rs=0.36; P<.001) and high-sensitivity troponin T (rs=0.23; P=.020). The other clinical variables, including ejection fraction, showed no significant associations. On multiple linear regression analysis, the only independent predictor was cystatin C concentration (P<.001; β=0.479; R=0.547) (Figure 1).
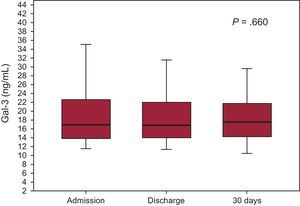
The median concentrations at discharge and at 30 days were 16.8 [14.01-22.1] and 17.6 [13.9-21.4] ng/mL and, as shown in Figure 2, the serial measurements analysis–on arrival at the emergency department, at discharge, and at 30 days–showed no differences (P=.660). Equally, there were no differences when we analyzed the percentage of patients with Gal-3 > 17.8 ng/mL: 46% at admission, 45% at discharge, and 46% at 30 days.
As an exploratory analysis, we evaluated the relationship between Gal-3 and prognosis. Over a median follow-up of 453 [156-1076] days, a total of 53 patients (48%) had an adverse event (25 died and 28 were readmitted). On univariable Cox regression analysis, Gal-3 at admission as a quantitative variable (per ng/mL, hazard ratio [HR], 1.70; 95% confidence interval [95%CI], 1.02-2.84; P=.042) or dichotomous variable (> 17.8 ng/mL, HR, 2.17; 95%CI, 1.25-3.77, P=.006) was associated with a higher risk of adverse events; however, after adjustment for renal function, this significance disappeared (P>.1).
In patients with chronic HF, an increased Gal-3 at 3 to 12 months has been found to confer increased mortality and hospitalization.3–5 In patients with acute HF, the only data available are those from the COACH study, in which patients with an increase > 17.8 ng/mL in Gal-3 at 6 months after discharge or a 15% relative increase from baseline had a higher risk of death and/or hospitalization due to HF.4 Our investigation is the first to study the behavior of Gal-3 in the short-term (the first 30 days) after an episode of acute HF. Admission concentrations were similar to those at discharge (median stay, 7 days) and at 30 days. Therefore, in patients hospitalized due to acute HF, Gal-3 concentrations show no dynamic changes from admission, and monitoring these values provides no additional information in the first month after discharge. This indicates that the underlying pathophysiological process is unmodifiable in the short-term. Gal-3 concentrations distinguish a phenotype of more severely ill patients, who are more likely to have a greater deterioration of renal function and a worse clinical outcome in the long-term, with more readmissions and higher mortality. The prognostic analysis was limited by the single-center nature and the small population of this study, but the findings confirm the high dependence of Gal-3 on renal function, as has previously been noted.6 Further studies are needed to ascertain the clinical usefulness of Gal-3 and its relationship to renal function.
FUNDINGThe reagents for this study were supplied by bioMérieux without charge.
CONFLICTS OF INTERESTD.A. Pascual-Figal has received fees for lectures from Biomerieux, Roche, and Novartis.