Somatic mutations causing clonal expansion of hematopoietic cells (clonal hematopoiesis of indeterminate potential [CHIP]) increase with age and are associated with a higher risk of developing a hematological malignancy.1 In the cardiovascular field, they have been found to be associated with atherosclerosis and inflammation.2 Limited information is available in chronic heart failure (HF).
We studied a consecutive cohort of 60 patients with chronic HF without a previous history of cancer. Baseline characteristics are detailed in table 1. The study was performed in accordance with the ethics committee of Germans Trias and Pujol Hospital and all patients signed an informed consent form. Targeted deep sequencing was performed on a custom panel including 43 myeloid- and CHIP-related genes, using deoxyribonucleic acid extracted from peripheral blood samples. Libraries were prepared using the SureSelect QXT capture chemistry (Agilent Technologies, United States) and sequenced on a HiSeq2500 following a 2 x 75bp paired-end reads standard protocol (Illumina, United States) at a mean depth of coverage of 2905-fold. Reads were aligned using BWA 0.7.12. Packages SAMtools 1.2 and VarScan 2.4.0 were used for variant calling without variant allele frequency (VAF) threshold. Sequencing and mapping errors were removed by discarding variants with a low mapping quality (< 20), variants located at highly variable regions, and variants occurring in ≥ 5% of the cohort. Synonymous variants and variants with a minor allele frequency> .01, according to available population databases, were also excluded. Statistical analysis was performed using the statistical package SPSS, version 23.0 (SPSS Inc, United States). We reviewed the largest reported CHIP cohorts to determine the prevalence of CHIP.
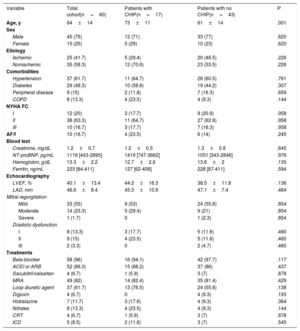
Clinical, biochemical, echocardiographic characteristics and treatment of the cohort (n=60) and between groups of patients presenting with CHIP (n=17) or without CHIP (n=43)
| Variable | Total cohort(n=60) | Patients with CHIP(n=17) | Patients with no CHIP(n=43) | P |
|---|---|---|---|---|
| Age, y | 64±14 | 73±11 | 61±14 | .001 |
| Sex | ||||
| Male | 45 (75) | 12 (71) | 33 (77) | .620 |
| Female | 15 (25) | 5 (29) | 10 (23) | .620 |
| Etiology | ||||
| Ischemic | 25 (41.7) | 5 (29.4) | 20 (46.5) | .226 |
| Nonischemic | 35 (58.3) | 12 (70.6) | 23 (53.5) | .226 |
| Comorbidities | ||||
| Hypertension | 37 (61.7) | 11 (64.7) | 26 (60.5) | .761 |
| Diabetes | 29 (48.3) | 10 (58.8) | 19 (44.2) | .307 |
| Peripheral disease | 9 (15) | 2 (11.8) | 7 (16.3) | .659 |
| COPD | 8 (13.3) | 4 (23.5) | 4 (9.3) | .144 |
| NYHA FC | ||||
| I | 12 (20) | 3 (17.7) | 9 (20.9) | .958 |
| II | 38 (63.3) | 11 (64.7) | 27 (62.8) | .958 |
| III | 10 (16.7) | 3 (17.7) | 7 (16.3) | .958 |
| AF/f | 10 (16.7) | 4 (23.5) | 6 (14) | .245 |
| Blood test | ||||
| Creatinine, mg/dL | 1.2±0.7 | 1.2±0.5 | 1.3±0.8 | .645 |
| NT-proBNP, pg/mL | 1116 [443-2895] | 1419 [747-3662] | 1051 [343-2846] | .976 |
| Hemoglobin, g/dL | 13.3±2.2 | 12.7±2.6 | 13.6±2 | .135 |
| Ferritin, ng/mL | 223 [84-411] | 127 [62-408] | 228 [87-411] | .594 |
| Echocardiography | ||||
| LVEF, % | 40.1±13.4 | 44.2±16.3 | 38.5±11.8 | .136 |
| LAD, mm | 46.6±8.4 | 45.3±10.9 | 47.1±7.4 | .464 |
| Mitral regurgitation | ||||
| Mild | 33 (55) | 9 (53) | 24 (55.8) | .854 |
| Moderate | 14 (23.3) | 5 (29.4) | 9 (21) | .854 |
| Severe | 1 (1.7) | 0 | 1 (2.3) | .854 |
| Diastolic dysfunction | ||||
| I | 8 (13.3) | 3 (17.7) | 5 (11.6) | .460 |
| II | 9 (15) | 4 (23.5) | 5 (11.6) | .460 |
| III | 2 (3.3) | 0 | 2 (4.7) | .460 |
| Treatments | ||||
| Beta-blocker | 58 (96) | 16 (94.1) | 42 (97.7) | .117 |
| ACEI or ARB | 52 (86.3) | 15 (88.2) | 37 (86) | .437 |
| Sacubitril/valsartan | 4 (6.7) | 1 (5.9) | 3 (7) | .878 |
| MRA | 49 (82) | 14 (82.4) | 35 (81.4) | .429 |
| Loop diuretic agent | 37 (61.7) | 13 (76.5) | 24 (55.8) | .138 |
| Digoxin | 4 (6.7) | 0 | 4 (9.3) | .193 |
| Hidralazine | 7 (11.7) | 3 (17.6) | 4 (9.3) | .364 |
| Nitrates | 8 (13.3) | 4 (23.5) | 4 (9.3) | .144 |
| CRT | 4 (6.7) | 1 (5.9) | 3 (7) | .878 |
| ICD | 5 (8.5) | 2 (11.8) | 3 (7) | .545 |
ACEI, angiotensin-converting enzyme inhibitor; AF/f, atrial fibrillation/flutter; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA FC, New York Heart Association functional class.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
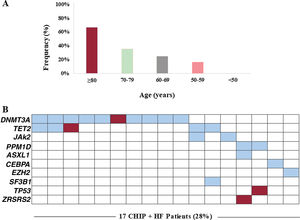
CHIP was found in 28% of patients with a total of 30 variants detected. Overall, 9 (15%) patients carried 1 mutation, 4 (7%) had 2 concurrent mutations, 3 (5%) had 3 mutations, and 1 (2%) patient had 4. Consistent with previous studies, the prevalence of CHIP increased with age: 67% in patients ≥ 80 years (n=8), 36% between 70 and 79 years (n=15), 25% between 60 and 69 years (n=16) and 17% between 50 and 59 years (n=12) (P=.01; figure 1A). Of note, no CHIP carriers were detected in patients <50 years (n=9). The mean number of mutations detected per patient also increased with age: 2.3 (≥ 80 years), 1.7 (70-79 years), 1.4 (60-69 years), and 1 (< 60 years). The frequency of CHIP in HF was considerably higher in all age groups than in previously published cohorts, including unselected populations as well as patients with coronary heart disease.2 However, this observation needs to be interpreted cautiously given that the prevalence of CHIP in the reported cohorts may have been influenced by the variety of sequencing technologies used in these studies, some of which did not reach the sensitivity reported for some of the variants detected in the current study. According to previous studies, the most frequently affected gene was DNMT3A (17%), followed by TET2 (8%). Experimental studies have reported that mutations in these 2 genes promote cardiac dysfunction in murine models of HF.3,4 Recurrent mutations were also detected in 2 patients (3%) in JAK2 and PPM1D, while individual mutations were identified in ASXL1, CEBPA, EZH2, SF3B1, TP53, and ZRSR2 (figure 1B). Median VAF was 1.96% with values ranging from 1.05% to 37.25%. Most variants (77%) had VAF values <5%. Interestingly, 3 patients carried a clone harboring known pathogenic myeloid mutations with a VAF larger than 20%, but showed no cytopenia or other hematological symptoms: DNMT3A p.R882H mutation (VAF 37%); a clone harboring both the DNMT3A p.R882H mutation (VAF 30%) and a nonsense TET2 mutation (VAF 30%); and a 27bp deletion in CEBPA (VAF 27%). Baseline clinical characteristics, hematological profiles and treatment did not differ in HF patients between CHIP carriers and noncarriers (table 1). The presence of DNMT3A mutations was associated with diastolic dysfunction (35% vs 16%, P=.031). Time of follow-up was 43±11 months, without differences between patients with and without CHIP (P=.500). There were four (23.5%) deaths in patients with CHIP and 7 (16.3%) in patients without CHIP (P=.513). The combined endpoint (HF hospitalization or death) occurred in 7 (41.2%) patients with CHIP and in 9 (20.9%) patients without CHIP (P=.110). CHIP was not related to death (hazard ratio [HR], 1.53; 95% confidence interval [95%CI], 0.45-5.24]; P=.497) or the combined endpoint (HR, 2.12; 95%CI, 0.79-5.71; P=.137) in a Cox regression analysis with proportional hazards. In patients with CHIP, there were no differences in clinical outcomes between patients with and without ischemic etiology (P=.861). There was no new-onset cancer during follow-up.
Prevalence of CHIP. A, prevalence of CHIP according to age. B, distribution of genes mutated in the cohort. Each column represents a patient, and each row represents a gene. Boxes colored in blue indicate presence of CHIP (red color indicates the presence of 2 or 3 mutations, respectively, in the same gene). HF, heart failure.
Contemporary registries show that currently a large percentage of HF patients die from noncardiac causes, mainly associated comorbidities and cancer. Moreover, recent studies indicate that HF patients are more prone to develop incident cancer,5 and therefore there is a need for a better understanding of the causal relationship between HF and tumor growth. Similarly, recent studies suggest that CHIP is a new causal risk factor for HF and is associated with poor outcomes.6
FUNDINGThis work was supported in part by Generalitat de Catalunya (Departament de Salut) PERIS Acció Instrumental de Programes de Recerca Orientats (SLT002/16/00234) and AGAUR (2017-SGR288 and 2017-SGR-483) financial support from CERCA Programme/Generalitat de Catalunya, Fundació Internacional Josep Carreras, Societat Catalana de Cardiologia, “la Caixa” Banking Foundation, and Red de Terapia Celular-TerCel (RD16/00111/0006), CIBER Cardiovascular (CB16/11/00403) projects, as a part of the Plan Nacional de I+D+I, and it was co-funded by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER). The research leading to this invention has received funding from “la Caixa” Foundation. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación (MCIN) and the Pro CNIC Foundation.
AUTHORS’ CONTRIBUTIONSL. Palomo performed sequencing studies, interpreted sequencing data and performed analysis; E. Santiago-Vacas collected samples, clinical data and performed analysis; D. Pascual-Figal and J.J. Fuster reviewed sequencing and statistical analyses; F. Solé and A. Bayés-Genis designed the study and provided samples. All authors reviewed the manuscript and gave final approval.
CONFLICTS OF INTERESTThe authors report no conflicts of interest.