In patients with heart failure (HF) and left ventricular systolic dysfunction, treatment with intravenous ferric carboxymaltose (FCM) and sodium-glucose co-transporter-2 inhibitors (SGLT2i) is associated with substantial clinical benefits.1 However, the mechanisms behind such benefits remain incompletely understood. Some preliminary findings suggest that SGLT2i may increase cell-iron availability.2 In the Myocardial-IRON trial, we found that in patients with HF and iron deficiency, FCM was associated with cardiac magnetic resonance (CMR) changes indicative of myocardial iron repletion (decrease in the T2* and T1 mapping sequences).3 The current analysis evaluated the association between treatment with FCM with early short-term changes in T2* and T1-mapping CMR sequences across baseline treatment with SGLT2i.
This is a posthoc analysis of a randomized, double-blind, placebo-controlled Myocardial-IRON trial. Inclusion and exclusion criteria are reported elsewhere but included patients with stable HF, left ventricular ejection fraction <50%, and iron deficiency.3 A total of 53 patients were randomized 1:1 to receive either FCM 1000mg (n=27) or placebo (n=26). CMR studies were performed by 2 experienced operators on a 1.5 Tesla magnetic resonance scanner using the spine and phased array 6-channel surface coils. A region of interest was chosen for T1 and T2* analysis in the mid-left ventricular septum. Detailed information on technical issues is published elsewhere.3 The endpoints were changes in myocardial iron content as measured by T2* and T1 mapping CMR sequences 7 and 30 days after FCM or placebo administration across treatment with empagliflozin. All patients gave written informed consent. The study conforms to the principles outlined in the Declaration of Helsinki and Good Clinical Practice of the International Conference on Harmonization. The study protocol was approved by the Agencia Española del Medicamento y Productos Sanitarios and by the Comité Etico de Investigación Cínica of Hospital Clínico Universitario de Valencia. All statistical comparisons were performed under the intention-to-treat principle. Linear mixed effect models were used to evaluate the endpoints. All analyses were adjusted for the baseline value of the regressed outcome, type 2 diabetes mellitus, and the interaction term treatment*visit (7- and 30-day). No adjustment was made for multiple comparisons. The linear mixed effect models are presented as least square means with their respective 95% confidence intervals and P values. All analyses were performed using STATA 15.1. A 2-sided P value of .05 was considered significant.
The median age was 73 years [interquartile range: 65 to 78], 40 (75.5%) were men, 29 (54.7%) were diabetic, 5 (9.4%) were on stable treatment with empagliflozin 10mg/d, and most of the patients (94.3%) were in New York Heart Association class II. At baseline, the median left ventricular ejerction fraction and NT-proBNP were 39% [33-47], and 1690 pg/mL [1010-2828], respectively. All patients exhibited iron deficiency. No significant differences in baseline characteristics were found across the treatment groups, including T2* and T1-mapping (table 1).
Baseline characteristics by treatment arm
| Variables | Placebo-no empagliflozin(n=23) | Placebo-empagliflozin(n=3) | FCM- no empagliflozin(n=25) | FCM- empagliflozin(n=2) | P |
|---|---|---|---|---|---|
| Demographics and medical history | |||||
| Age, y | 71 [67-79] | 67 [59-77] | 73.5 [64-77] | 72 [65.5-79] | .886 |
| Male sex | 16 (69.6) | 3 (100) | 19 (76) | 2 (100) | .821 |
| Hypertension | 16 (69.6) | 3 (100) | 20 (80) | 2 (100) | .745 |
| Dyslipidemia | 13 (56.5) | 3 (100) | 16 (64) | 2 (100) | .435 |
| Diabetes mellitus | 11 (47.8) | 3 (100) | 13 (52) | 2 (100) | .260 |
| Former smoker | 3 (13) | 1 (33.3) | 3 (12) | 0 | .635 |
| Coronary artery disease | 9 (39.1) | 1 (33.3) | 11 (44) | 2 (100) | .472 |
| Admission for AHF in last year | 15 (65.2) | 1 (33.3) | 15 (60) | 1 (50) | .763 |
| COPD | 4 (17.4) | 2 (66.7) | 7 (28) | 0 | .263 |
| NYHA functional class | .435 | ||||
| II | 23 (100) | 3 (100) | 22 (88) | 2 (100) | |
| III | 0 | 0 | 3 (12) | 0 | |
| KCCQ, points | 68 [54-90] | 91 [31-94] | 74 [63-92] | 80 [75-84] | .858 |
| Vital signs | |||||
| Heart rate, bpm | 68 [65-77] | 64 [58-108] | 74 [70-82] | 68.5 [67-70] | .522 |
| SBP, mmHg | 126 [113-148] | 122 [118-146] | 116 [109-130] | 139.5 [125-154] | .224 |
| Electrocardiogram | |||||
| Atrial fibrillation | 11 (47.8) | 3 (100) | 9 (36) | 1 (50) | .200 |
| LBBB | 5 (21.7) | 1 (33.3) | 6 (24) | 0 | 1.000 |
| CMR parameters | |||||
| LVEF, % | 36 [30-46] | 33 [31-38] | 44 [38-49] | 41 [32-49] | .384 |
| T1mapping at baseline, ms | 1068 [1030-1116] | 1101 [1082-1152] | 1082 [1062-1106] | 1108.5 [1037-1180] | .196 |
| T2* mapping at baseline, ms | 38 [30-42] | 34 [33-77] | 40 [36-45] | 37 [34-40] | .527 |
| Laboratory | |||||
| Hemoglobin, g/dL | 13.4 [12.1-14.4] | 14.6 [13.1-14.8] | 13.0 [11.9-13.3] | 13.3 [13.1-13.4] | .204 |
| Transferrin saturation, % | 14.9 [9.6-19.0] | 21.9 [9.6-22.0] | 15.0 [12.0-19.2] | 16.0 [15.7-16.2] | .863 |
| Ferritin, ng/mL | 47.1 [23.0-131.0] | 48.4 [30.0-65.0] | 77.0 [56.0-126.0] | 60.0 [58.0-62.0] | .292 |
| eGFR, mL/min/1.73 m2 | 64.3 [48.9-80.0] | 49.7 [46.8-9.2] | 59.4 [50.0-71.2] | 72.7 [50.4-95.0] | .867 |
| NT-proBNP, pg/mL | 1180 [1010-2849] | 1990 [601-2527] | 1990 [976-2830] | 2255 [1728-2781] | .871 |
| Seric sodium, mmol/L | 141 [140-142] | 137 [135-144] | 140 [140-142] | 141 [140-142] | .633 |
| Seric potassium, mmol/L | 4.6 [4.4-4.9] | 4.6 [3.9-4.7] | 4.7 [4.2-5.0] | 4.4 [4.3-4.5] | .627 |
AHF, acute decompensated heart failure; CMR, cardiac magnetic resonance; COPD, chronic pulmonary obstructive disease; eGFR, estimated glomerular filtration rate assessed by Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation; KCCQ, Kansas City Cardiomyopathy Questionnaire; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal propeptide brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure.
Values are expressed as No. (%) or [percentile 25% to percentile 75%].
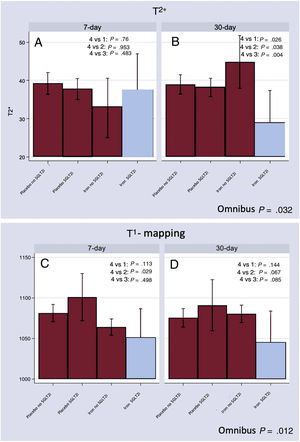
At follow-up, T2* differed across treatment groups (omnibus P value=.032). At 7 days, no differences were found (figure 1A). At 30 days, those receiving FCM on treatment with empagliflozin showed significantly lower 30-day T2* [Δ-15.8ms (−26.5 to −5.1; P=.004)] compared with the FCM-no empagliflozin group (figure 1B). At this same time frame, T2* values remained lower when compared with the other treatment categories (figure 1B).
T2* and T1 mapping CMR changes across treatment groups. A: T2* CMR changes at 7 days.. B: T2* CMR changes at 30 days. C: T1 mapping CMR changes at 7 days. D: T1 mapping CMR changes at 30 days. Group 1: placebo without empagliflozin; Group 2: FCM without empagliflozin; Group 3: placebo with empagliflozin; Group 4: FCM with empagliflozin.
CMR, cardiac magnetic resonance; FCM, ferric carboximaltose.
Likewise, T1-mapping significantly differed across treatment groups (omnibus P value=.012). At 7 days, patients in the FCM-empagliflozin group exhibited lower T1-mapping; however, the difference was only significant compared with those who received placebo and were on treatment with empagliflozin (figure 1C). At 30 days, compared with the FCM-no empagliflozin group, patients in the FCM-empagliflozin group exhibited a statistical trend to lower T1-mapping values (figure 1D).
In this posthoc analysis, we found that in patients with stable HF with left ventricular ejerction fraction <50% and iron deficiency, myocardial iron repletion following FCM was greater in patients on baseline treatment with empagliflozin. Recently, in a subanalysis of the EMPATROPISM trial Santos-Gallego et al., 4 suggested that treatment with empagliflozin may increase myocardial iron repletion. Specifically, they found a decrease in T2* evaluated by CMR after 6 months of treatment with SGLT2i compared with placebo and without iron supplementation. Ferritin levels were equally significantly reduced, and soluble transferrin receptor was increased in the empagliflozin arm.4
Prior and current data reinforce the hypothesis that SGLT2i seems to increase the myocardial availability of iron. Although there is no definitive explanation for this phenomenon, a study by Ghanim H et al.5 reported that dapagliflozin causes hepcidin suppression, one of the main proteins involved in iron homeostasis, which avoids iron release from storage sites and which is increased in proinflammatory states, such as HF. In the same study, dapagliflozin also increased plasma concentrations of transferrin and the expression of transferrin receptors 1 and 2, responsible for the entry of iron into the cardiomyocyte.5
The current report has some limitations. First, it is a nonprespecified subgroup analysis of a small clinical trial. Second, the number of patients on treatment with empagliflozin was low, and all of them had type 2 diabetes, which may provide considerable uncertainty about the current findings. Further studies are warranted.
FUNDINGThis work was supported in part by an unrestricted grant from Vifor Pharma, CIBERCV [grant numbers 16/11/00420 and 16/11/00403], Unidad de Investigación Clínica y Ensayos Clínicos INCLIVA Health Research Institute, Spanish Clinical Research Network (SCReN; PT13/0002/0031 and PT17/0017/0003), cofounded by Fondo Europeo de Desarrollo Regional—Instituto de SaludCarlos III, and Proyectos de Investigación de la Sección de Insuficiencia Cardiaca 2017 from Sociedad Española de Cardiología.
AUTHORS’ CONTRIBUTIONSM. Lorenzo and R. de la Espriella contributed equally.
M. Lorenzo and R. de la Espriella were responsible for drafting the manuscript as well as preparing the tables and figures. I. Cardells was responsible for monitoring the patients during the study and for data collection. J.L. Górriz and A. Bayés-Genís have critically reviewed the manuscript and contributed to the correction of errors and suggestions from the reviewers. J. Núñez is responsible for devising the working hypothesis, statistical analysis and review of the different versions of the manuscript.
CONFLICTS OF INTERESTJ. Núñez has received board speaker fees and travel expenses from Novartis, Roche Diagnostics, Abbott, Rovi, Vifor Pharma, Novo Nordisk, Boehringer Ingelheim, and AstraZeneca (modest). A. Bayés-Genís has received board membership fees and travel expenses from Novartis, Roche Diagnostics, Vifor Pharma, and Critical Diagnostics (modest). The remaining authors have no disclosures to report.