Patients with Fontan circulation (FC) have a high incidence of clinical complications. However, no biomarker is able to accurately stratify risk. The aim of this study was to analyze the relationship between biomarkers and clinical complications, including carbohydrate antigen 125 (CA125) for the first time, and to propose a risk estimation based on a combination of biomarkers.
MethodsCross-sectional study of patients with FC. The clinical endpoint was the combination of heart failure, atrial arrhythmias, veno-venous fistulae, protein-losing enteropathy, or plastic bronchitis. Demographic, clinical, and laboratory variables were analyzed, including CA125, NT-proBNP, renal and liver function, and red cell distribution width (RDW). We performed univariate and multivariate analyses of the relationship between these variables and the composite endpoint. Cutoff values were calculated by ROC curves.
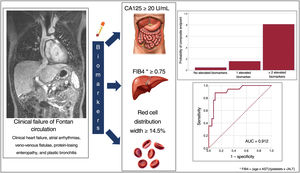
ResultsWe included 56 patients (27.4±7.8 years). A total of 34% showed the composite endpoint, with significantly higher CA125 levels (30.1 IU/mL vs 12.6 IU/mL; P=.001). In the multivariate model, the biomarkers related to the endpoint were LnCA125 (OR, 5.1; 95%CI, 1.2-22), RDW (OR, 1.8; 95%CI, 1.1-3.1), and FIB4 (OR, 38, 95%CI, 1.7-855). The cutoff points were CA125 ≥ 20 U/mL, FIB4 ≥ 0.75, and RDW ≥ 14.5%, and the probability of the occurrence of the endpoint was 81% if ≥ 2 biomarkers were elevated.
ConclusionsCA125 elevation is associated with a higher prevalence of complications in patients with Fontan-type circulation. CA125 levels ≥ 20U/mL, FIB4 ≥ 0.75 and RDW ≥ 14.5% identify with a high probability the clinical failure of FC.
Keywords
The Fontan procedure is a palliative surgical technique for the treatment of functionally univentricular congenital heart disease when biventricular repair is not possible.
The procedure connects the systemic venous circulation directly to the pulmonary arteries. This connection may be by means of a direct communication of the right atrium to the pulmonary arteries (classic or atriopulmonary technique), part of the right atrium (lateral tunnel), or a prosthetic conduit (extracardiac conduit). The Fontan circuit creates an in-series circulation, characterized by a low cardiac index and high central venous pressure. In the long-term, these patients have a high risk of complications such as heart failure, arrhythmias, thromboembolism, cyanosis secondary to veno-venous fistulae, protein-losing enteropathy, plastic bronchitis, liver disease, and venous insufficiency.1,2
Unfortunately, the biomarkers that are generally used in patients with biventricular function are not well established in patients with Fontan circulation. Several studies have evaluated the usefulness of different biomarkers such as galectin-3,3 endothelin-1,4 red cell distribution width (RDW),5 brain natriuretic peptide (BNP), and N-terminal pro-brain natriuretic peptide (NT-proBNP)6–9 for follow-up monitoring and identifying patients with an increased risk of complications.
Recently, the usefulness of the biomarker carbohydrate antigen 125 (CA125) in patients with heart failure has been demonstrated, providing better identification of congestive patterns.10 As many of the complications in patients with a Fontan circulation are due to a significant degree of systemic venous congestion, CA125 could be of particular use in this group of patients.
The aim of the present study was to analyze the association between the biomarkers currently available in routine clinical practice and the presence of clinical complications in patients with a Fontan circulation, including the analysis of CA125 for the first time in this patient group. A secondary aim was to propose an estimation of risk of clinical failure of Fontan circulation based on a combination of biomarkers.
METHODSStudy populationThis was a cross-sectional, single-center study of a cohort of consecutive patients with Fontan circulation, with prospective recording of the study variables during outpatient clinic follow-up at a national referral unit for adult congenital heart disease.
Variables analyzedThe variables analyzed included demographics, type of congenital heart disease, surgical technique, and date of Fontan procedure. In all patients, we measured the marker CA125 (Abbott Alinity, chemiluminescent microparticle immunoassay, Abbott Laboratories, USA) and parameters of hepatic and renal function, iron profile, and full blood count, including RDW. Using these values, we calculated the liver fibrosis indices FIB4 (fibrosis 4), APRI (AST to Platelet Ratio Index), and MELD-Na (Model for End-stage Liver Disease-Na).
The presence of atrioventricular and aortic valve regurgitation was determined on transthoracic echocardiography, and ventricular function was estimated using cardiac magnetic resonance (performed within the previous 2 years). Ventricular dysfunction was classified as severe if ventricular ejection fraction was <35%, moderate if 35%-45%, and mild if 45%-55%.
Data were recorded on New York Heart Association (NYHA) functional class and clinical complications: clinical heart failure, sustained atrial arrythmias (atrial fibrillation or atrial flutter), veno-venous fistulae with oxygen saturation ≤ 90%, protein-losing enteropathy, and plastic bronchitis. Lastly, we recorded patients’ treatment at the time of inclusion.
Statistical analysisThe primary clinical endpoint was defined as the composite of the following factors: presence of clinical heart failure (defined as dyspnea with NYHA ≥ II and congestive signs of malleolar edema, ascites or diuretic treatment), sustained atrial arrhythmia (atrial fibrillation or atrial flutter), veno-venous fistula with oxygen saturation ≤ 90% in the absence of fenestration, protein-losing enteropathy, or plastic bronchitis. The biomarkers used were NT-proBNP, CA125, FIB4, APRI, RDW, platelet count, and glomerular filtration rate.
The normality of distribution of the quantitative variables was analyzed using the Kolmogorov-Smirnov test.
Variables with a normal distribution are expressed as mean±standard deviation, and variables with a nonnormal distribution are expressed as median [interquartile range].
Logarithmic transformation was performed for variables with nonnormal distribution to optimize their analysis and graphic representation.
Using a univariate analysis (chi-square test for categorical variables, Student t test for variables with normal distribution, and Mann-Whitney U test for variables with nonnormal distribution), we studied the association between the clinical, laboratory, and ventricular function parameters with the presence of the composite endpoint. P <.05 was considered statistically significant. We also analyzed the association of each of the analyzed variables with the composite endpoint using a bivariate logistic regression model. With this model, we graphically analyzed the functional relationship between the biomarkers that were significant and the probability of having an unfavorable clinical profile.
A multivariate analysis was carried out (Forward LR, adjusted for the time in years since Fontan procedure) including the variables with a statistically significant association (P <.05) on the previous univariate analysis.
To estimate the probability of having an unfavorable clinical profile, the selected continuous variables were dichotomized, taking cutoff values based on the optimal sensitivity and specificity on the ROC curve. With these variables and cutoff points, we created a second multivariate logistic model (Forward LR). Using an ROC curve, we studied the estimation capacity of the model, and the probability of having an adverse clinical profile was represented as a function of all of the variables analyzed.
Informed consent and ethical considerationsThe study was performed in accordance with the Declaration of Helsinki and was assessed and approved by the hospital ethics committee (registration number 2021-892-1). The patients were appropriately informed and signed an informed consent form prior to their inclusion.
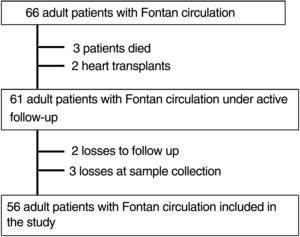
RESULTSPatient characteristics and clinical eventsAs shown in figure 1, of the 66 patients with Fontan circulation who were seen in the national referral unit for adult congenital heart disease, 56 were included in the analysis (3 died, 2 had heart transplants, there were 2 losses to follow-up, and 3 losses at sample collection).
The general characteristics of the patients included in the sample are described in table 1. The mean age at the time of inclusion was 27.4±7.8 years, with a balanced distribution by sex. Only 5 patients (8.9%) had an atriopulmonary Fontan circulation. Most remained in a good functional class (69.6% of patients were in NYHA I), with normal or mildly reduced ventricular systolic function in 78.5%. Regarding prescribed treatment, 13 patients (23.2%) required diuretics, 8 (14.3%) were on pulmonary vasodilators, and 16 (28.5%) were on anticoagulants.
Demographic and clinical characteristics and treatment received for the whole series and for patients with favorable or unfavorable clinical profile
| All (n=56) | Unfavorable clinical profile (n=17) | Favorable clinical profile (n=39) | P | |
|---|---|---|---|---|
| Age, y | 27.4±7.8 | 32.6±9.6 | 25.2±5.7 | .004 |
| Time since Fontan procedure, y | 19.9±7.7 | 24.2±9.1 | 17.9±6.3 | .001 |
| Women | 29 (51.8) | 7 (41.2) | 22 (56.4) | .294 |
| Diagnosis | ||||
| Tricuspid atresia | 20 (35.7) | 5 (29.4) | 15 (38.5) | .349 |
| Double-inlet left ventricle | 8 (14.3) | 2 (11.8) | 6 (15.4) | |
| Pulmonary atresia | 7 (12.5) | 2 (11.8) | 5 (12.8) | |
| Unbalanced AV canal | 4 (7.1) | 0 | 4 (10.3) | |
| Mitral atresia | 3 (5.4) | 2 (11.8) | 1 (2.6) | |
| Single ventricle of indeterminate morphology | 4 (7.1) | 1 (5.9) | 3 (7.7) | |
| Other | 10 (17.8) | 5 (29.4) | 5 (12.8) | |
| Left ventricle morphology | 40 (71.4) | 11 (64.7) | 29 (74.5) | .334 |
| Fontan type | ||||
| Atriopulmonary | 5 (8.9) | 5 (29.4) | 0 | <.001 |
| Lateral tunnel | 14 (25) | 6 (35.3) | 8 (20.5) | |
| Extracardiac | 37 (66.1) | 6 (35.3) | 31 (79.5) | |
| Cyanotic patients | 5 (9) | 4 (24) | 1 (2.6) | .028 |
| NYHA | <.001 | |||
| I | 39 (69.6) | 4 (23.5) | 35 (89.7) | |
| II | 15 (26.8) | 11 (64.7) | 4 (10.3) | |
| III-IV | 2 (3.6) | 2 (11.8) | 0 | |
| Medical treatment | ||||
| ACEI/ARB | 7 (12.5) | 4 (23.5) | 3 (7.7) | .099 |
| Beta-blockers | 11 (19.6) | 7 (41.2) | 4 (10.3) | .007 |
| Antiarrhythmics | 6 (10.7) | 5 (29.4) | 1 (2.6) | .003 |
| Diuretics | 13 (23.2) | 8 (47.1) | 5 (12.8) | .005 |
| MRA | 10 (17.9) | 7 (41.2) | 3 (7.7) | .003 |
| Pulmonary vasodilator | 8 (14.3) | 5 (29.4) | 3 (7.7) | .033 |
| Anticoagulation | .001 | |||
| Acenocoumarol | 6 (10.7) | 4 (23.5) | 2 (5.1) | |
| Direct acting anticoagulant | 10 (17.8) | 6 (35.3) | 4 (10.3) | |
| ASA | 40 (71.5) | 7 (41.2) | 33 (84.6) | |
| Ventricular function | .149 | |||
| Normal to slightly reduced | 44 (78.6) | 10 (58.8) | 34 (87.2) | |
| Moderately reduced | 5 (9.4) | 2 (11.8) | 3 (7.7) | |
| Severely reduced | 4 (7.5) | 3 (17.6) | 1 (2.6) | |
| Ventricular ejection fraction, % | 52.1±9.8 | 46.9±10.1 | 54.1±9.1 | .015 |
| Moderate to severe atrioventricular valve insufficiency | 5 (9.4) | 3 (17.6) | 2 (5.1) | .065 |
| Moderate to severe aortic valve insufficiency | 2 (3.6) | 0 | 2 (5.1) | .063 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; AV, atrioventricular; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association functional class.
Values are expressed as No. (%) or mean±standard deviation.
Seventeen patients (30.4%) met at least 1 of the criteria included in the composite endpoint: clinical heart failure (12 patients, 21.4%), atrial arrhythmia (6 patients, 10.7%), veno-venous fistula with oxygen saturation ≤ 90% (4, 7.1%), and protein-losing enteropathy (3 patients, 5.4%).
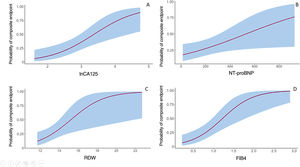
Study of carbohydrate antigen 125 and other biomarkersThe biomarker CA125 had a nonnormal distribution in this study cohort, with a median of 15.4 [8.1-32.7] IU/mL. CA125 values were significantly higher in patients with the composite event: 30.1 [21.1-57.4] vs 12.6 [7.9-18.7] IU/mL (P=.001). Figure 2A shows the probability of having a composite event as a function of the logarithmic transformation of CA125 values.
Of the rest of the proposed biomarkers (NT-proBNP, FIB4, APRI, RDW, platelet count, and glomerular filtration rate), all apart from glomerular filtration rate showed statistically significant differences in the patient group who had the composite event (table 2). The probability of having an event according to values of NT-proBNP, FIB4, and RDW is presented in figure 2B-D.
Blood parameters for the whole series and by clinical profile
| All (n=56) | Unfavorable clinical profile (n=17) | Favorable clinical profile (n=39) | P | |
|---|---|---|---|---|
| Creatinine, mg/dL | 0.8±0.2 | 0.8±0.1 | 0.8±0.2 | .919 |
| Glomerular filtration rate, mL/min/1.73 m2 | 108.8±16.2 | 102.8±14.7 | 109.9±16.8 | .409 |
| Hemoglobin, g/dL | 15.7±1.8 | 15.1±2.6 | 15.9±1.3 | .174 |
| Platelets, ×109 | 186.1±72.3 | 141.1±52.5 | 205.7±71.4 | .001 |
| AST, U/L | 24.8±6.4 | 24.7±6.4 | 24.8±6.5 | .978 |
| ALT, U/L | 26.8±11.6 | 25.9±11.2 | 27.2±11.9 | .705 |
| Iron, μg/dL | 86.4±46.4 | 91.4±73.7 | 84.3±28.8 | .615 |
| Transferrin saturation index, % | 24.4±13.9 | 29.9±21.6 | 22.1±8.6 | .079 |
| Ferritin, ng/mL | 90.4±106.6 | 85.5±84.3 | 92.6±116.5 | .834 |
| RDW, % | 13.8±2.3 | 15.3±3.5 | 13.1±0.8 | .02 |
| MELD-Na | 6.6±4.2 | 8.4±5.4 | 5.9±3.6 | .123 |
| FIB4 | 0.9±0.5 | 1.3±0.6 | 0.7±0.3 | .002 |
| APRI | 0.4±0.17 | 0.5±0.2 | 0.3±0.1 | .002 |
| NT-proBNP, pg/mL | 205.7±215.8 | 306.3±239.8 | 164.5±193.5 | .047 |
| CA125, U/mL | 20.3±19.1 | 33.2±27.2 | 14.7±10.4 | .001 |
ALT, alanine aminotransferase; APRI, Aspartate aminotransferase to Platelet Ratio Index; AST, aspartate aminotransferase; NT-proBNP, N-terminal pro-brain natriuretic peptide.
Values are expressed as mean±standard deviation.
The time in years since the Fontan procedure was performed was longer in patients who had an event (24.2±9.1 vs 17.9±6.3; P=.001). Patients who had undergone an atriopulmonary technique and those with a lower ventricular ejection fraction had a higher prevalence of clinical complications, but no significant differences were observed according to ventricular morphology or type of heart disease. Other variables analyzed, such as functional status and anticoagulant treatment, also showed a difference in distribution between patients with the composite event and those without. However, both were related to the endpoint definition.
Multivariate modelTable 3 shows the results of the multivariate analysis of the variables that reached statistically significant differences in the univariate analysis, adjusted for time in years since the Fontan procedure.
Results from the univariate analysis and multivariate analysis adjusted for time since Fontan procedure
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| LnCA125 | 4.7 (1.7-12.8) | .002 | 5.1 (1.2-22) | .03 |
| NT-proBNP, pg/mL | 1.0 (1-1.006) | .035 | — | — |
| EF, % | 0.92 (0.8-0.99) | .023 | — | — |
| FIB4 | 13.9 (2.9-65) | .001 | 38 (1.7-855) | .02 |
| Platelets, ×109 | 0.98 (0.96-0.99) | .002 | — | — |
| MELD-Na | 1.15 (0.99-1.34) | .07 | — | — |
| Hemoglobin, g/dL | 0.75 (0.55-1.05) | .09 | — | — |
| ARPI | 245 (5-11.795) | .005 | — | — |
| RDW, % | 1.75 (1.13-2.74) | .013 | 1.8 (1.1-3.1) | .03 |
| Years since Fontan procedure | 1.12 (1.03-1.23) | .008 | 1.02 (0.87-1.19) | .8 |
95%CI, 95% confidence interval; ARPI, Aspartate aminotransferase to Platelet Ratio Index; EF, ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; OR, odds ratio; RDW, red cell distribution width.
In this model, only LnCA125 (odds ratio [OR]=4.7; 95% confidence interval [95%CI], 1.7-12.8; P=.002), RDW (OR=1.75; 95%CI, 1.13-3.1; P=.013), and FIB4 (OR=13.9; 95%CI, 2.9-65; P=.001] maintained their association with the occurrence of the composite event.
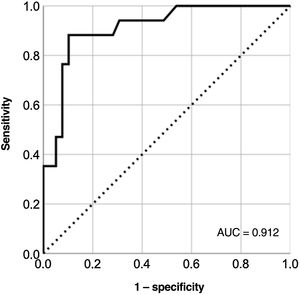
Model to identify unfavorable clinical profile based on biomarkersBased on the results of the multivariate model, we calculated the cutoff points with the optimal sensitivity and specificity for LnCA125 (≥ 3, corresponding to CA125 ≥ 20 U/mL), FIB4 (≥ 0.75), and RDW (≥ 14.5%) according to the ROC curves for each variable (figure 1 of the supplementary data). With these cutoff values, we created a multivariate model (table 1 of the supplementary data) with a good capacity for identifying patients with an adverse clinical profile (area under the curve=0.91) (figure 3 and figure 4).
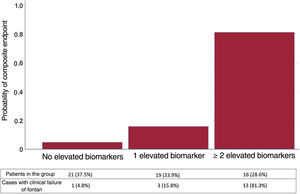
We estimated the probability of a pattern of clinical failure of Fontan circulation as a function of the values obtained above the proposed threshold for the different biomarkers (LnCA125, FIB4, and RDW). Figure 5 shows that patients with all 3 biomarkers below the cutoff point rarely had a composite event (5%), while those with 2 or more biomarkers above the cutoff had a very high probability (81%) of having an unfavorable clinical profile.
DISCUSSIONThis study shows for the first time that adult patients who have undergone a Fontan procedure and go on to develop complications have higher CA125 levels, indicating that this biomarker could be useful in the assessment of Fontan circulation failure, in which venous congestion plays a key role. Furthermore, we propose an estimation of the risk of having an unfavorable profile based on 3 biomarkers that are easy to measure in clinical practice (CA125, RDW, and FIB4).
Clinical endpointsPatients with Fontan circulation have a unique hemodynamic pattern characterized by the diversion of the systemic venous circulation to connect directly to the pulmonary arteries, without a functional subpulmonary ventricle, meaning they have a low cardiac output and a high central venous pressure. Failure of Fontan circulation can occur in different pathological situations that cause dysfunction of the single ventricle or increase the systemic venous pressure. In this context, the clinical expression of Fontan circulation failure can manifest in very different ways, such as heart failure, arrhythmias, cyanosis from veno-venous fistulae, protein-losing enteropathy, and plastic bronchitis.
In our series, 30% of the patients had signs or symptoms compatible with Fontan circulation failure, similar to the reported rates in contemporary series.3,11 The clinical characteristics were also very similar to those recorded in our population, with mean ages around 25 years, predominantly left single-ventricle morphology, and good functional class, with 70% of the patients in NYHA I. However, our series had a lower incidence of atrial tachyarrhythmias (11% vs 20%), which can probably be explained by the lower prevalence of atriopulmonary interventions (less than 10% of our patients).
Biomarkers: risk stratificationGiven the high incidence of complications and the high variability and complexity of the pathophysiological manifestations of Fontan circulation failure, the follow-up of this patient group represents one of the greatest challenges of care for adult congenital heart disease units. In the latest European guidelines on congenital heart disease,12 the threshold for performing cardiac catheterization in the context of complications has been lowered, but it is not considered a routine diagnostic test for stable patients. In recent years, multiple biomarkers have been studied in this patient group, with the aim of obtaining diagnostic and prognostic information in the least invasive way possible,13–18 but results have been varied and there is currently no recommended marker for the monitoring of these patients.
In our study, thrombocytopenia, high NT-proBNP, RDW, and CA125 levels, and FIB4 and APRI liver fibrosis scores were associated with a worse clinical profile, but only RDW, CA125, and FIB4 were associated with Fontan circulation failure in the multivariate model.
One of the most important points in this study concerns CA125. Although this marker has traditionally been used for monitoring and risk stratification in ovarian cancer, it has recently been associated with heart failure, primarily with congestion.19 However, there has been limited study of this biomarker in the field of congenital heart disease. In congenial heart disease with left-to-right shunt, it has been observed that high levels of CA125 could be used to predict the development of pulmonary hypertension (PHT) and heart failure at follow-up.20,21 Although the pathophysiological mechanism remains to be defined, one of the more plausible theories suggests that its release into the bloodstream may be caused by the activation of mesothelial cells located mainly in the pleura, pericardium, and peritoneum in response to the combination of mechanical (increased venous pressure) and inflammatory stimuli.19 Therefore, given that many of the complications associated with Fontan circulation are due to increased systemic venous pressure and secondary congestion, it would be of great interest to perform studies to validate this biomarker as an indirect marker of congestion and confirm its prognostic value. One point for discussion would be the cutoff value for predicting complications. Most laboratories use a cutoff of 35 U/mL, but this value has been used mainly in the context of oncological disease. In our study, the cutoff was 20 U/mL, very similar to that reported in a recent study22 in which levels<23 U/mL identified a subgroup of patients with low risk of death or rehospitalization following discharge from a hospital stay due to heart failure.
Although the more established and frequently-used biomarkers in the field of heart failure are the natriuretic peptides, and in our patients, we observed an association between high values of NT-proBNP and clinical complications, this marker did not maintain a good capacity for discriminating the composite endpoint in the multivariate model. This is in line with the disparate results observed in other studies.7,23 Natriuretic peptides mainly reflect situations of ventricular dysfunction, with increased wall stress related to pressure and volume overload. This pathophysiological response is less common in patients with Fontan circulation, who by definition have a considerably reduced preload.
RDW is a measure of the variation in size of red blood cells (anisocytosis). It is a parameter that is usually included as part of a full blood count and its normal value is between 11% and 14%. Values above 14% have been associated with higher mortality in heart failure.24 The elevation of this biomarker is associated mainly with situations of deficiency and inflammatory processes,25 factors that increase the incidence of complications and mortality13 in this patient group.
In patients with Fontan circulation, it has been reported5 that RDW values >14.5% are independently correlated with high central venous pressure and low cardiac output. In our study, the cutoff value above which the greatest number of clinical complications occurred was also >14.5%.
Liver disease is a recognized complication and is often the first manifestation of Fontan circulation failure. The increase in systemic venous pressure causes congestion of the splanchnic venous circulation and disorders of the lymphatic circulation. The effects of this on the liver can range from hepatic congestion to progressive fibrosis with portal hypertension and liver cirrhosis.26
In the assessment of such liver disease, blood parameters are of great importance. Elevated GGT and alkaline phosphatases are the most common abnormalities, although these have not been associated with a state of fibrosis or low cardiac output. Thrombocytopenia is a common finding in situations of hypersplenism due to portal hypertension or increased central venous pressure. In our study, it was also associated with increased risk of events. In patients with advanced liver disease, the MELD scale is very useful for prognosis. However, in patients with Fontan circulation who often receive anticoagulant treatment, this scale loses its value. In our study, we analyzed the MELD-Na score and did not observe an association with increased risk of events.
Although the use of the FIB4 and APRI scales as markers of fibrosis and advanced liver disease in this population has been recognized,27 differences were not observed when scores were compared with liver biospies.28 These results can be explained by the fact that both scales use very high cutoff values, extrapolating the results observed in other types of liver disease. Patients with Fontan circulation are much younger and have a different pathophysiology. Furthermore, both scales include the platelet count, which can be reduced due to other causes, such as chronic cyanosis. In our study, both markers were associated with worse clinical outcome, and FIB4 was shown to be a biomarker with great predictive value, but using a cutoff value of > 0.75, considerably lower than the > 1.4529 described in patients with liver disease not related to Fontan circulation.
Multivariate risk stratification modelLastly, one of the novel points of this article is the combined use of 3 biomarkers (CA125, FIB4, and RDW) with a high discriminatory ability for the occurrence of the composite event. Patients with all 3 biomarkers lower than the proposed threshold could be categorized as low risk, while those with 2 or 3 raised biomarkers had a high probability of having one of the clinical complications described. It would be very interesting to evaluate the utility of this model in future prospective multicenter studies that take into account clinical events and hemodynamic variables.
LimitationsThe present study has several limitations. First, those inherent to a cross-sectional study, in which the biomarkers are measured at one point in time without taking into account changes over time and without following up patients with complications such as transplant or death. The composite endpoint was clinical, and although the definition of heart failure in these patients is debatable, it has been associated with poor prognosis in previous publications.30 Furthermore, the sample was small, albeit a considerable number of patients for this type of disease from a single center.
Second, in the multivariate analysis, we did not include the Fontan technique used, because in our center, atriopulmonary connection is rarely performed. However, the model was adjusted for time since Fontan procedure, a variable that in all studies is associated with increased incidence of events.
Third, given the small sample size, the predictive model was created using the complete dataset and was not tested in a validation sample. It would be desirable to perform a multicenter study in the near future that would provide more data and establish a training sample and a validation sample.
CONCLUSIONSIn adult patients with a univentricular heart who have undergone Fontan surgery, elevated CA125 is associated with increased prevalence of clinical complications.
Patients with CA125 levels ≥ 20 U/mL, FIB4 ≥ 0.75, and RDW ≥ 14.5% have a very high probability of Fontan circulation failure. This model should be validated in future prospective studies and in relation to hemodynamic variables and occurrence of clinical events.
FUNDINGThis study had no source of funding.
AUTHORS’ CONTRIBUTIONSAll the authors contributed to the present study. F. Buendía Fuentes and J. Rueda Soriano contributed primarily to the initial concept and the study protocol, as well as writing and supervising the article. P. Jover Pastor and S. Lozano Edo contributed to the writing of the project for the ethics committee and data collection. M.A. Arnau Vives performed the statistical analysis. M. Rodríguez Serrano, J. Aguero, and A. Osa Sáez contributed to writing and editing the article. I. Conde Amiel and V. Aguilera Sancho-Tello advised on the variables to include in the analysis and contributed to editing the article. L. Martínez Dolz supervised and edited the article.
CONFLICTS OF INTERESTNone.
- –
Patients with a functional single ventricle who undergo a Fontan procedure have a high risk of clinical complications.
- –
The biomarkers that are generally used in patients with biventricular physiology are not well established in patients with Fontan circulation.
- –
CA125has been demonstrated to be useful in patients with heart failure, providing better identification of congestion patterns.
- –
In patients with Fontan circulation, it would be of great use to have a noninvasive means to provide diagnostic and prognostic information.
- –
This study identifies CA125 as a useful biomarker for the first time in patients with a Fontan circulation.
- –
A risk estimation is proposed, based on 3 biomarkers that are easy to measure in clinical practice (CA125, RDW, and FIB4) to identify patients with an unfavorable clinical profile.
- –
Cutoff values are presented for these 3 biomarkers (CA125 ≥ 20 U/mL, FIB4 ≥ 0.75, and RDW ≥ 14.5%) that allow good characterization of the risk of clinical complications.
- –
The presented model should undergo external validation in future prospective multicenter studies.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.05.029