Phospholamban is an inhibitor of the sarcoplasmic calcium pump, which regulates contractility and relaxation. Mutations in its gene, PLN, have been associated with aggressive phenotypes of both dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy.1,2
Herein we report a family diagnosed with arrhythmogenic cardiomyopathy with some peculiar features, carrying a Dutch founder mutation in phospholamban (PLN c.40_42delAGA; p.Arg14del).3 The genotype-phenotype correlation allowed the identification of some red flags, which should lead to suspicion of this mutation in the clinical work-up.
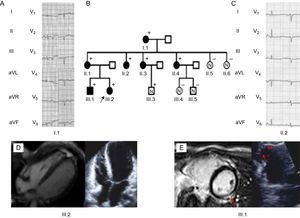
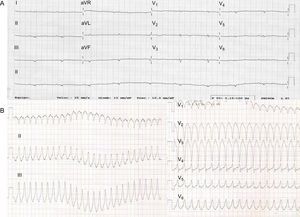
The proband (III.2, Figure 1) was a 28-year-old woman with a past medical history of presyncopes. The electrocardiogram (ECG) showed QS inferiorly and striking low voltages throughout all leads (Figure 2).
A and C: Electrocardiograph of the carriers: Low voltages and poor R wave progression are the hallmark. Notice the inverted T waves in let precordial leads. B: Family tree. The black arrow points to the proband (III.2). + represent carriers. Filled black figures represent affected carriers. D: The proband's echocardiogram and cardiac magnetic resonance revealed normal biventricular volumes, severe biventricular dysfunction, and absence of late gadolinium enhancement. E: Cardiac imaging and echocardiographic findings of III.1. Red arrows indicate left ventricle lateral late gadolinium enhancement patch. On the left, a right ventricle aneurysm of the free wall is observed.
Echocardiography showed a nondilated left ventricle with global hypokinesia and a left ventricular ejection fraction of 40%. Cardiac magnetic resonance with gadolinium did not show late enhancement. The right ventricle was not dilated and had global normal systolic (right ventricular ejection fraction of 51%) function, although the apex was remarkably hypokinetic.
Blood tests were unremarkable. A coronary computed tomography scan showed normal coronaries. The results of 24-hour Holter monitoring were normal. The patient was discharged on beta-blockers, an angiotensin-converting enzyme inhibitor, and spironolactone.
She was readmitted for a new collapse 3 months later. During that admission, sustained ventricular tachycardia (Figure 2) was documented, prompting implantation of an implantable cardioverter-defibrillator. The patient remained in New York Heart Association (NYHA) functional class I. Genetic analysis ruled out pathogenic mutations in the 5 desmosomal genes, LMNA and MYBPC3. However, a pathogenic mutation in PLN, p.Arg14del, was identified. There was no other relevant family history of cardiomyopathy or sudden death.
Cascade family screening identified 7 additional carriers of the PLN p.Arg14del mutation:
- 1.
An asymptomatic 25-year-old brother (III.1, Figure 1) had frequent (>1000/24h) ventricular ectopics. His ECG showed late R wave transition in precordial leads. Although the echocardiogram was normal, cardiac magnetic resonance revealed a hypokinetic right ventricle apex and a subepicardic late gadolinium enhancement patch in the lateral wall of the left ventricle. Biventricular systolic function was normal. Recurrent episodes of symptomatic nonsustained ventricular tachycardia (15-20 beats) were documented. Beta-blockers were started and an implantable cardioverter-defibrillator was implanted.
- 2.
The proband's 52-year-old mother (II.1) was asymptomatic. Her ECG showed late R transition in the precordial leads. The results of echocardiogram and cardiac magnetic resonance were normal.
- 3.
The maternal grandmother (I.1) was incidentally diagnosed at the age of 74 years in a preoperative cardiac evaluation. Her ECG demonstrated negative T waves in inferior and lateral leads. The echocardiogram showed a left ventricular ejection fraction of 45% with normal-sized left ventricular diameters. An exercise echocardiogram was negative for ischemia, and Holter monitoring failed to demonstrate any arrhythmia. She had a good response to medical treatment with left ventricular ejection fraction normalization.
- 4.
Three asymptomatic maternal aunts (II.2, II.3, and II.4) showed poor R wave progression and flat T waves throughout the ECG. The results of echocardiograms and cardiac magnetic resonance were normal
- 5.
A 16-year-old cousin (III.3) was asymptomatic and the clinical work-up was normal.
Due to the morphology of ventricular tachycardia and ECG abnormalities and their status as carriers, 2 patients met the criteria for definitive arrhythmogenic right ventricular cardiomyopathy whilst 3 had a borderline diagnosis.
Haplotype analyses of markers around PLN, in 2 affected Spanish PLN mutation carriers, were compared with the Dutch series. Interestingly, the Spanish patients shared 4 out of 5 markers from the shared Dutch haplotype, suggesting a common founder ancestor.
In the family presented herein, the penetrance of the disease is 6/8 (75%). Because PLN p.Arg14del is a pathogenic mutation, this family is a clear example of the variability of the clinical phenotype in relatives with arrhythmogenic right ventricular cardiomyopathy.4 Of note, recent evidence shows a trend for female carriers to show milder phenotypes than males yet they nevertheless show malignant ventricular arrhythmias.5 A notable finding was the wide variety of ECG abnormalities found in the family: widespread low QRS voltage (even reminiscent of restrictive cardiomyopathy) in the proband, inverted T waves in inferolateral leads (typical of arrhythmogenic left ventricular cardiomyopathy), and poor R wave progression in all but 1 carrier.
Low voltage and poor R wave progression in ECG is a red flag in arrhythmogenic right ventricular cardiomyopathy or dilated cardiomyopathy patients and should lead to suspicion of a PLN mutation as a cause of the disease. This is paramount, particularly when assessing the timing of implantable cardioverter-defibrillator implantation, which, in view of the current data, should be indicated before the patient has severely depressed systolic dysfunction.5