Coarctation of aorta (CoA) accounts for between 7% and 10% of cases of congenital heart disease. In this letter, we focus on a special and very uncommon type (1%-5% of all coarctations), complete aortic occlusion. This condition is characterized by the total absence of distal flow, but with luminal continuity between the ascending and descending aorta, and thus differs from interrupted aortic arch, in which this continuity does not exist. It normally occurs in cases of untreated long-standing CoA, which progress to complete occlusion (distal to the origin of left subclavian artery).1–3
The diagnosis of this condition is usually established in the context of the study of hypertension, stroke, or heart failure. From the anatomical point of view, it is usually accompanied by extensive collateral circulation, and the aortic wall shows cystic degeneration of the medial layer, as well as marked loss of structure, which can trigger complications such as aneurysms and dissections, or even aortic rupture. Surgical treatment is associated with a high rate of morbidity, with serious complications such as paraplegia. For this reason, percutaneous treatment of this disorder has become highly relevant in recent years.3,4
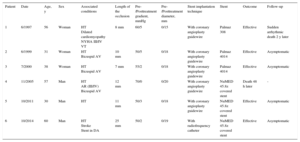
We present 6 adult patients (3 women and 3 men, with a mean age of 45.3±13 years [range, 30-60 years]). From the clinical point of view, all of them had hypertension, 3 also had congenital aortic valve disease (bicuspid aortic valve), and 1 had dilated cardiomyopathy with severe systolic dysfunction, and was in New York Heart Association functional class III (Table).
Clinical Characteristics, Technical Features, Type of Stent, and Follow-up
| Patient | Date | Age, y | Sex | Associated conditions | Length of the occlusion | Pre-/Posttreatment gradient, mmHg | Pre-/Posttreatment diameter, mm | Stent implantation technique | Stent | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6/1997 | 56 | Woman | HT Dilated cardiomyopathy NYHA III/IV VT | 8 mm | 60/5 | 0/15 | With coronary angioplasty guidewire | Palmaz 308 | Effective | Sudden arrhythmic death 2 y later |
| 2 | 6/1999 | 31 | Woman | HT Bicuspid AV | 10 mm | 50/5 | 0/18 | With coronary angioplasty guidewire | Palmaz 4014 | Effective | Asymptomatic |
| 3 | 7/2000 | 38 | Woman | HT Bicuspid AV | 7 mm | 55/2 | 0/18 | With coronary angioplasty guidewire | Palmaz 4014 | Effective | Asymptomatic |
| 4 | 11/2005 | 57 | Man | HT AR (III/IV) Bicuspid AV | 12 mm | 70/0 | 0/20 | With coronary angioplasty guidewire | NuMED 45.8z covered stent | Death 48 h later | - |
| 5 | 10/2011 | 30 | Man | HT | 11 mm | 50/3 | 0/18 | With coronary angioplasty guidewire | NuMED 45.8z covered stent | Effective | Asymptomatic |
| 6 | 10/2014 | 60 | Man | HT Stroke Stent in DA | 25 mm | 50/2 | 0/19 | With radiofrequency catheter | NuMED 45.8z covered stent | Effective | Asymptomatic |
AD, anterior descending artery; AR, aortic regurgitation; AV, aortic valve; HT, hypertension; NYHA, New York Heart Association; VT, ventricular tachycardia.
All of them underwent a percutaneous intervention to gain access to the coarcted segment and implant a stent—standard Palmaz (Cordis) in the first 3 patients and, from 2005 onward, expanded polytetrafluoroethylene (ePTFE)-covered stents. The procedure was performed under general anesthesia, using a dual arterial access (radial or humeral plus femoral). In the first 5 patients, the occluded segment was crossed using a Hi-Torque Cross-It 200 (Abbott) or Asahi Confianza (World Medical) guidewire via the radial/humeral access and, once this had been accomplished, the distal portion of the guidewire was snared with a loop catheter (Amplatz GooseNeck), and the guidewire was exteriorized by way of a radial-femoral “monorail”. The sixth patient required the use of a radiofrequency catheter (Nikannen) via femoral access to penetrate the occluded segment, given its length (approximately 20mm), which could not be crossed using an angioplasty guidewire.
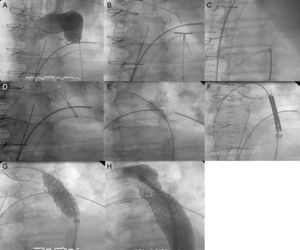
Once the radial-femoral monorail had been established, dilatation was performed progressively with 1-mm to 4-mm-balloons inserted via the upper access to achieve distal flow; at that moment, an Amplatz extra-stiff guidewire was introduced via the femoral access, to continue the dilatation with larger-caliber balloons (BALT 8-10mm). Finally, the stent, which was mounted on a balloon (NuMED Z-Med or BIB balloon) with a diameter similar to that of the aorta, was implanted at the level of the subclavian artery. Then, the distal portion of the stent was postdilated using a larger-caliber balloon to achieve correct apposition. The results were confirmed both angiographically and hemodynamically (Figure).
A: angiography from the upper extreme showing complete occlusion with no distal flow; B: crossing of the occlusion with angioplasty guidewire (Cross-it 300) via radial access; C: guidewire capture using a GooseNeck loop snare and establishment of the radial-femoral monorail; D: dilatation by coronary balloon angioplasty via the radial access; E: dilatation with a larger-caliber balloon (BALT) via the femoral access; F: introduction of the Mullins sheath with the stent; G: stent implantation; and H: postdilatation for correct apposition of the stent to the wall.
The femoral access was closed using the Prostar XL closure device (Abbott) in all the patients, and there were no vascular complications in any of the patients. The patients were discharged from the hospital on the third day and received antiplatelet therapy with aspirin 100mg/d for 3 to 6 months.
Elimination of the occlusion and stent implantation were achieved in 100% of the patients. We observed a reduction in the gradient from 55±8mmHg to 3±2mmHg (P<.001). There was only 1 serious complication in a patient (patient 4) who had extensive collateral circulation and a giant aneurysm in an intercostal artery. He died 48hours after the procedure due to hypovolemic shock secondary to massive hematemesis. The autopsy demonstrated that the stent had lost its covering (for no clear reason), thus leaving a shunt between the intercostal aneurysm and descending aorta, which, with the increase in pressure beyond the point of the coarctation, provoked retrograde flow in the aneurysm that caused a rupture in the esophageal wall and produced an aortoesophageal fistula.4
The mean follow-up was 8.4 (2-17) years (involving office visits and diagnostic imaging techniques—ultrasound and computed tomography or magnetic resonance). There were no aneurysms, dissections, or evidence of recoarctation of the aorta. The mortality rate has increased to 33% (the aforementioned patients 4 and 1, who had dilated cardiomyopathy with severe systolic dysfunction and died 2 years later of sudden arrhythmic death).
Our series of 6 patients undergoing stent implantation for totally occluded CoA is comparable to other published series with good immediate and long-term results, as this condition is associated with a high mortality rate. The use of ePTFE-covered stents can be recommended in this type of complex intervention in calcified aortas with a loss of structure due to medial cystic necrosis, which can progress to the formation of aneurysms, dissections, or even rupture. The stent should be positioned correctly in the wall with its ePTFE covering in order to reduce the incidence of complications. To simplify the procedure, after ensuring the expansion of the stent with its ePTFE covering, we now employ the Nudel premounted system (Evomed), very similar to that of the Melody pulmonary valve prosthesis, which might have prevented the fatal complication in patient 4.
In our experience, totally occluded CoA represents 4% of the total number of percutaneous interventions in CoA and 12% of cases involving stent implantation. The collateral circulation is usually highly developed, which confers special surgical difficulty and risk on the procedure. This has led to the development of the percutaneous approach, with techniques for crossing the occluded segment using coronary angioplasty or radiofrequency guidewires and implantation of a stent, which should preferably be covered with ePTFE, as it reduces long-term complications.5,6