Keywords
INTRODUCTION
Currently, aortic stenosis is the most frequent valvular disease in Europe.1 Patients with symptomatic severe aortic stenosis (SAS) have an average survival of 2 years, and valve replacement surgery is the only effective treatment.2,3 However, high surgical risk means that surgery is not viable in approximately 30%-40% of patients with SAS.4
Patients with SAS deemed ineligible for surgery have low survival rates and very limited quality of life. Percutaneous valvuloplasty emerged as a possible treatment for these patients, but it was found to have a relatively poor outcome.5 Recently, aortic valve prostheses implanted through a catheter have been developed and now represent an alternative for SAS patients who are not suitable for surgery.6-9 We report initial experience with PIAVP (percutaneous implantation of aortic valve prosthesis) in our center.
METHODS
Technique
Two types of prostheses for PIAVP are currently marketed: the Edwards-Sapiens balloon-expandable, 22-24 Fr catheter,6-8 and the Core-valve self-expandable 18 Fr catheter.9 The Core-valve has been reported to be associated with a higher risk of stroke and requires a permanent pacemaker. At our center, the Edwards-Sapiens implant is used, and the techniques referred to here refer to that alternative.
The procedure can be performed transfemorally or transapically, the latter being reserved for cases when the anatomy of the aortoiliac system precludes the transfemoral approach.8 Aortography and computed tomography (CT) are used to evaluate this aspect. In the 4 patients described here, transfemoral implantation was used.
A 14 Fr introducer sheath is inserted via the femoral artery allowing percutaneous valvuloplasty to be performed (20´30 mm balloon). Dilators of 16, 18, 20, and 22 Fr are then introduced, followed by the prosthesis catheter.
The Edwards-Sapiens prosthesis (Edwards Lifesciences Inc., Irvine, California, USA) is a stainless steel slotted tube attached to 3 bovine pericardium valves. It is available in diameters of 23 and 26 mm, which need 22 and 24 Fr catheters for implantation, respectively. Before insertion, the valve is folded over a 30 mm balloon. It then moves on a 0.35" guidewire through the iliac artery, the abdominal aorta, the descending aorta, the aortic arch and the ascending aorta before insertion into the left ventricle ("retrograde" approach). The catheter uses a system that allows the device to be angled for changes in the direction of the thoracic aorta, thus avoiding damage to the aortic wall and minimizing the risk of embolic stroke.
The proper positioning of the stent prior to deployment is essential, and fluoroscopy, echocardiography, and aortography are used for this. Once the stent is positioned correctly, a temporary pacemaker is implanted during overstimulation (180-220 lat/min) to prevent its displacement. If later echocardiographic scans show that the stent is correctly inserted, the guidewire and catheters are removed.
Intravenous heparin (70 IU/kg) is administered during the procedure. Aspirin and clopidogrel are administered prior to the intervention and are continued indefinitely for 1-6 months after the intervention at doses of 100 mg/day and 75 mg/day, respectively.
Inclusion Criteria
At present, candidates for PIAVP are those with SAS, with symptoms attributable to the condition, and who are ineligible for surgery. Currently, patient and/or cardiologist preferences for the treatment alone are not considered an indication for the intervention. In general, rejection for surgery should be due to having a EuroSCORE index >20% and/or an STS (Society of Thoracic Surgeons) index >10%.
Definitions
We evaluated outcomes and events at 30 days (death, myocardial infarction, cardiac surgery, stroke, vascular complications). Aortic valve ring, ejection fraction, aortic insufficiency, transaortic gradients, and valve area were assessed before and after the procedure using transthoracic and transesophageal echocardiography. The procedure was considered a success if the patient survived the operation and the prothesis was implanted correctly and functioned normally (≥30% decrease in transaortic gradients and the absence of severe aortic insufficiency).
RESULTS
All patients were elderly (mean age [range], 81 [77-84] years) and male (Table). Co-morbidities included heart disease, kidney failure, recent heart attack, chronic bronchopathy, and severe pulmonary hypertension. Mean EuroSCORE and STS index values were 23% and 24%, respectively.
The procedure was performed under general anesthesia and tracheal intubation. Inotropics were administered as required to ensure maximal stability in patients' hemodynamic condition.
In 2 patients, the entire procedure was performed percutaneously (closure was with two 10 Fr Prostar per patient). In the remaining 2 patients, the artery was surgically exposed and then surgically closed after the procedure. Subsequently, patients were transferred to the cardiac resuscitation unit, where they were extubated.
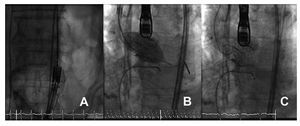
The prosthesis was implanted successfully in all patients, and no significant aortic insufficiency or alterations of the coronary ostium were recorded (Figure).
Figure. Different time-points of the intervention are shown. A: the folded prosthesis moving through the aortoiliac system. B: implantation of the prosthesis. Note that at this point the temporary pacemaker is used to produce overstimulation at a frequency of 180-220 beats/min to generate a systolic blood pressure of £60 mm Hg. C: the implanted prosthesis.
No complications arose during hospitalization after the procedure, except for a groin hematoma without significant anemia. The last of the patients was in hemodialysis for chronic kidney failure and had chronic slow atrial fibrillation before percutaneous insertion of the aortic prosthesis. A few days after the prosthesis was inserted, the patient was fitted with a pacemaker, though this was not attributed to a secondary obstruction due to PIAVP.
After 30 days, evolution was still event-free, and all patients had improved by at least 1 functional class.
DISCUSSION
Mean survival in symptomatic SAS is 2 years (1 year if heart failure is present) and surgery is the only treatment that improves prognosis and quality of life.2,3 In spite of this, one third of patients are considered unsuitable for surgery because of high surgical risk.4 Percutaneous valvuloplasty emerged over 2 decades ago as a therapeutic alternative for these patients, but it is rare to achieve an area >0.8 cm2 and valve restenosis means that medium term outcomes are poor.5
Interventional cardiologists have long dreamt of using catheters to implant aortic valves,10 but it was the possibility of joining biological valves to a metallic stent which finally allowed the procedure to be performed. It was initially performed in animals,11 and Bonhoeffer et al12 introduced prosthetic valves—in the pulmonary valve—for the first time in humans. The first percutaneous prosthesis for the aortic valve was implanted in humans 6 years ago, more than a decade after the initial experiences in animals.
In 2004, Cribier et al6 published details of their first experience with 6 patients. Of these, 1 died during the procedure due to stent migration during implantation, whilst in the other 5 cases the valve area increased to 1.7 cm2 and gradients were significantly reduced. In this initial experience, the prosthesis was introduced by the antegrade approach. The main advantage of this approach is that it uses the femoral vein rather than the artery, thus decreasing vascular complications and making it possible to perform the procedure under local anesthetic. However, the antegrade approach is technically more complex and can cause complications in the mitral valve and interatrial short-circuits. Currently, the retrograde approach described by Webb et al is used.7 In 50 patients with SAS and a EuroSCORE of 28%, success was achieved in 86%, with a perioperative and 30 day mortality rate of 2% and 12%, respectively. An interesting aspect of this study is that outcomes improved in the final 25 patients compared to the first 25. This reflects improvements along the learning curve, which likely affects not only technical aspects but also patient selection.
Later series included patients treated using the retrograde approach. In the REVIVE-I registry, which continued into RECAST, the prosthesis was implanted successfully in 27 (82%) of 33 patients. Currently, the prosthesis has been implanted in some 1000 patients worldwide using the transfemoral approach. In REVIVE-II, 106 patients (mean EuroSCORE, 30%) received the implant using the transfemoral route; implantation was successful in 88%, with a 30 day mortality of 13.2%, myocardial infarction in 8.5%, neurological events in 2.8%, and vascular complications in 13%. The randomized PARTNER study, which will look at 2 patient groups, is currently in the patient inclusion phase. The first group (n=650) includes patients with relatively high surgical risk but who are accepted for surgery, and will be randomized to either surgery or PIAVP using the Edwards prosthesis. Those in the second group (350 patients considered ineligible for surgery) will be randomized to PIAVP or medical treatment.
Currently, complications related to vascular access represent the primary limitation of PIAVP. Other complications are significant perivalvular failure due to inadequate expansion of the prosthesis, and stroke. In the future, it is likely that prostheses requiring smaller diameter catheters will be designed. This would facilitate the procedure and reduce the risk of vascular complications. Some experience has already been gained with a prosthesis that can be repositioned before being released,13 an approach which could lower the risk of displacement and poor positioning. On the other hand, information on the durability of the prosthesis from long-term studies is lacking. In vitro studies have shown that the prosthesis continues to function after more than 200 million heartbeats, which is equivalent to more than 5 years of life. Currently, some patients are still alive 5 years after the prosthesis was implanted, and the device has not shown signs of dysfunction.
In conclusion, our initial experience shows that PIAVP represents an alternative therapy for some patients with SAS in whom surgery is not an option.
ACKNOWLEDGEMENTS
We would like to thank Drs Thierry Lefevre and Eulogio García, for overseeing the procedures described; María Paños and Ana San Juan, nurses in the Interventional Cardiology Department; Drs Isidro Gómez-Moreno and Luis Suárez, of the Cardiovascular Anesthesia and Resuscitation Department; Drs María José Mesa and Ulises Ramírez, of the Cardiac Surgery Department; Dr Mar Moreno, of the Cardiac Imaging Department; Dr Luis Riera, of the Vascular Surgery Department; Drs Esteban López de Sá and David Dobarro, of the Cardiology Department; and Isabel Muñoz, of Edwards Lifesciences.
Correspondence: Dr. R. Moreno.
Unidad de Cardiología Intervencionista. Servicio de Cardiología. Hospital La Paz.
P.o de la Castellana, 261. Hospital General, planta 1.a diagonal. Madrid. España.
E-mail: raulmorenog@terra.es
Received August 28, 2008.
Accepted for publication September 12, 2008.