Many patients with severe aortic stenosis never undergo surgical treatment for various reasons. Apart from the standard risks, some patients face an additional problem: their carrying of a mechanical mitral valve. In these patients, transcatheter aortic valve implantation is a therapeutic option. The literature contains only few reports of this procedure being performed (usually transapically) in such patients. This paper reports the cases of 3 patients with severe aortic stenosis, all carrying a mechanical mitral valve and at high surgical risk, all of whom were implanted by transcatheter aortic valve implantation with an Edwards aortic valve prosthesis. All procedures were successful with no complications encountered.
Keywords
.
INTRODUCTIONSince Cribier1 performed the first transcatheter aortic valve implantation (TAVI) procedure in a patient in 2002, it has been used worldwide to implant more than 30 000 of the two types of aortic valve prosthesis available. The procedure is mainly indicated in patients with severe aortic stenosis who cannot undergo surgery or who are at very high surgical risk2–which becomes even worse if a patient has been implanted with a mechanical mitral prosthesis. The literature contains only few reports of such patients undergoing transcatheter implantation–usually transapically–of an Edwards aortic valve prosthesis.3, 4 The present paper records our experience of the use of TAVI in 3 patients carrying a mechanical mitral valve (Table 1).
Table 1. Selected Characteristics of the Present Patients, All of Whom Carried a Mechanical Mitral Prosthesis and Who Underwent Transcatheter Aortic Valve Implantation.
| Patient 1 | Patient 2 | Patient 3 | |
| Age (years) | 71 | 83 | 74 |
| Sex | Female | Female | Female |
| Logistic EuroSCORE (%) | 19.7 | 38 | 25 |
| Aortic valve area (cm2) | 0.5 | 0.5 | 0.6 |
| Mitroaortic distance (mm) | 7.3 | 7.3 | 7 |
| Postimplantation dysfunction of the mitral prosthesis | No | No | No |
| Aortic regurgitation | None | Trivial | Trivial |
This retrospective, observational study involved the period between January and December 2010, and included all patients with a mechanical mitral prosthesis who underwent TAVI at our center.
RESULTS Patient 1This patient was a 71 year-old woman who had undergone a commissurotomy 25 years earlier. Four years prior to the present procedure she received an ATS 29 mitral valve and was implanted with a tricuspid annuloplasty ring. At 3 years prior to the present procedure she received a VVI pacemaker owing to complete atrioventricular block. At 4 months prior to the present procedure she was diagnosed with severe aortic stenosis, and classified within functional class III-IV. An echocardiogram returned the following results: area of aortic valve 0.5cm2, ventricular dysfunction (ejection fraction 30%), pulmonary hypertension, aortic ring 21mm. The patient's EuroSCORE was 19.7%, and she was rejected for valve substitution surgery. Coronary angiography revealed no significant lesions. Computed tomography (CT) revealed the distance between the aortic ring and mitral prosthesis to be 7.3mm. The minimum caliber of the iliofemoral artery was also 7.3mm (Figure 1). Aortic valvuloplasty (transfemoral) was performed using a 23mm balloon catheter and a 26 mm Edwards SAPIEN XT valve implanted, both procedures guided by transesophageal echocardiography and fluoroscopy. Follow-up coronary angiography and echocardiography showed there to be no significant gradient nor regurgitation. The vascular access was closed successfully using the Prostar closure device; no complications were encountered. Clinical assessment at 30 days classified the patient within functional class I.
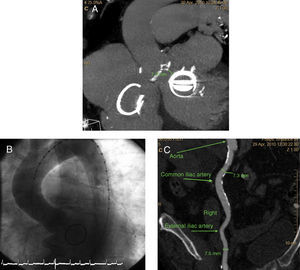
Figure 1. A: cardiac computed tomography showing the distance between the aortic ring and the mitral valve. B: aortogram prior to transcatheter aortic valve implantation. C: angiotomography of the iliac artery, showing the right common iliac artery to have a diameter of 7.3 mm.
Patient 2This patient was an 83 year-old woman who had received a St. Jude mechanical mitral prosthesis in 1999. She suffered chronic atrial fibrillation. Transesophageal echocardiography returned the following results: valve area 0.5cm2, pulmonary hypertension 75mmHg, ejection fraction 65%, aortic ring 18 mm, mitral prosthesis functioning normally. CT returned a distance between the aortic ring and the mitral prosthesis of 7.3 mm (Figure 2). The patient's EuroSCORE was 38%. A 23mm Edwards SAPIEN XT valve was successfully implanted by TAVI (transfemoral), guided by transesophageal echocardiography and fluoroscopy. The patient progressed without complications. At the 1-3 month check-up the patient was classified in functional class I.
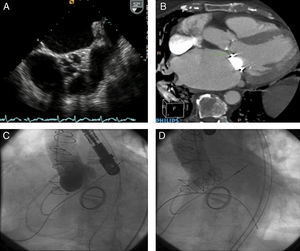
Figure 2. A: transesophageal echocardiogram showing severe aortic valve calcification and a reduced aperture. B: Cardiac computed tomography showing the distance between the aortic ring and mitral valve. C: aortogram prior to transcatheter aortic valve implantation.. D: aortogram following transcatheter aortic valve implantation showing a normally functioning aortic prosthesis.
Patient 3This patient was a 74 year-old woman with high blood pressure, dyslipidemia, diabetes type II, diabetic kidney disease, and kidney failure. She had undergone a mitral commissurotomy in 1978, and received a St. Jude mitral valve prosthesis in 2005. She suffered chronic atrial fibrillation. Her functional class had been deteriorating, and after ruling out a dysfunction of the mitral prosthesis she was referered for evaluation for possible TAVI. She was rejected for surgery (EuroSCORE 25%). Coronary angiography showed no stenosis. Transesophageal echocardiography returned the following results: systolic blood pressure 80mmHg, aortic ring 22mm, valve area 0.6 cm2. The mitroaortic distance was 7 mm.
A 26mm Edwards SAPIEN XT prosthesis was implanted by TAVI (transfemoral), guided by transesophageal echocardiography and fluoroscopy. Arterial access was closed using the Prostar closure device. Coronary angiographic monitoring showed extravasation of the contrast dye; internal compression was therefore performed using an 8mm balloon catheter (contralateral route) for 10min. Extravasation persisted after the balloon was deflated; an Advante V12 (10×38 mm) drug-eluting stent was therefore implanted. The next day the patient suffered complete atrioventricular block that required the implantation of a VVI pacemaker. After this no further problems were suffered in this respect. Assessment at one month classified the patient in functional class II. An echocardiogram showed the aortic prosthesis to be functioning normally with a mean gradient of 5mmHg and no aortic regurgitation. The left ventricular ejection fraction was 60%.
DISCUSSIONCarrying a mitral prosthesis is a serious additional problem to be taken into account when contemplating aortic valve substitution.3 Initially, TAVI with an Edwards valve was deemed contraindicated in patients with a mitral prosthesis, and those with such valves were excluded from the PARTNER study.5 It was considered that the rigidity of the prosthetic mitral ring and the short distance between the aortic ring and the mitral prosthesis might impede the adequate expansion of an aortic prosthesis, interfere with the correct functioning of the mitral prosthesis, or produce a ″watermelon seed″ effect,3, 6 with embolization of the aortic prosthesis. To avoid this embolization, the following precautions should be taken: a) prior to the procedure an exhaustive study should be made of the mitroaortic area, using transesophageal echocardiography to examine the aortic valve, the mitral prosthesis, and the distance between them; b) the behavior of the balloon should be monitored during valvuloplasty, paying special attention to how it inflates, its displacement, and any residual waist; c) a projection should be chosen that clearly shows the mitroaortic distance to facilitate the correct positioning of the prosthesis (e.g., a left anterior oblique projection with cranial inclination); d) inflation should be performed slowly to check for and correct any undesired movement of the prosthesis, and e) transesophageal echocardiography should be used to help position the aortic prosthesis correctly, to check its expansion, and to assess its functioning.
It is also important to know the characteristics and, in particular, the profile of the mechanical mitral prosthesis. Figure 3E and F shows some of the most commonly used metallic mitral prosthesis models. The profile of the mitral prosthesis conditions the percutaneous implantation of the aortic prosthesis. In patient 1, the ATS valve (which has a larger profile than the St. Jude valves previously implanted in patients 2 and 3) demanded a greater distance be present between the mitral prosthesis and the aortic ring. There is no consensus on an ideal distance that would allow the implantation of an aortic valve without problems, although a space of 3mm between the lower edge of the aortic ring and the upper edge of the mitral prosthesis ought to be sufficient when using the transapical route.7 In the present patients, which were treated via the transfemoral route, this distance was >7 mm.
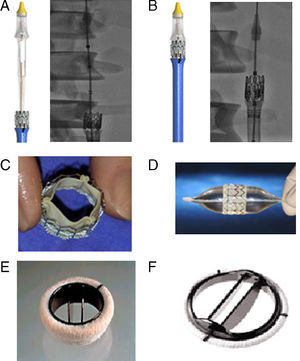
Figure 3. A: an Edwards SAPIEN XT prosthesis introduced before mounting on the balloon catheter. B: Edwards SAPIEN XT mounted on a balloon catheter (valve between the marks). C: an Edwards SAPIEN prosthesis. D: Edwards SAPIEN prosthesis mounted on a balloon catheter. E: an ATS Medical® mechanical prosthesis. F: a St. Jude Regent® mechanical prosthesis.
Few studies have been published on the implantation of an Edwards valve via the transfemoral route in patients carrying a mechanical mitral valve prosthesis.7, 8, 9 Some consensus exists in that a reduction in the movement of the prosthesis experienced when using this route would favor its choice, and indeed the introduction of the new Edwards SAPIEN XT prosthesis and Novaflex delivery system has almost eliminated the upward movement of the valve, allowing the prosthesis to be positioned in the desired place (Figure 3). One of the problems that could occur in these patients is the abnormal hemodynamic behavior of the mitral prosthesis caused by the low (with respect to the aortic ring) implantation of the aortic prosthesis, especially when the CoreValve type is used and it penetrates to a certain extent into the ventricle. In the present patients, echo-Doppler monitoring after the procedure and during follow-up revealed normal hemodynamic behavior for both valves.
The new Novafelx delivery system allows the use of an 18 Fr sheath for a 23mm prosthesis, and a 19 Fr sheath for a 26mm prosthesis. The procedure can also be performed in a completely percutaneous manner, making use of the Prostar closure device, system.
In conclusion, transfemoral TAVI with an Edwards SAPIEN XT prosthesis can be performed safely and effectively in patients carrying a mechanical mitral prosthesis. The new SAPIEN XT model, along with the Novaflex delivery system, allows the procedure to be performed in a fully percutaneous manner.
CONFLICTS OF INTERESTNone declared.
Received 21 January 2011
Accepted 13 February 2011
Corresponding author: Servicio de Cardiología, Hospital Clínico San Carlos, Profesor Martín Lagos s/n, 28040 Madrid, Spain. ejgarcia1@telefonica.net