Atrioventricular conduction disorders can appear after the implantation of percutaneous aortic CoreValve® prostheses in patients requiring permanent pacemakers (40%-45% of these patients). The aim of our study was to detect if 2- or 3-dimensional echocardiographic variables could predict the appearance of atrioventricular conduction disorders. For this purpose, the relationship of the prosthesis with the interventricular septum was studied in 26 consecutive patients. Twelve patients (46.1%) developed atrioventricular conduction disorders. A prosthetic penetration >12 mm in the left ventricular outflow tract and a contact surface >90% between the interventricular septum and the stent of the prosthesis in diastole were strongly associated with the appearance of conduction disturbances (87.5%; P=.034). The percentage of the prosthetic stent in contact with the interventricular septum in end diastole was the only independent predictor of atrioventricular conduction disorders (odds ratio=1.12; 95% confidence interval, 1.01-1.25; P=.03). The results suggest that a higher implantation of the prosthesis and a reduced stent length might decrease the incidence of this complication.
Keywords
.
IntroductionThe implantation of aortic biological prostheses using a percutaneous approach is an alternative to conventional surgical techniques for aortic valve stenosis in high-risk patients.1 The CoreValve® prosthesis (Medtronic-CorevalveTM, LLC; Minneapolis, Minnesota, United States) is one of the devices used for this purpose. The characteristics of the native valve, perivalvular tissues, and the prosthesis to be implanted can all influence the success of the procedure.2 The diameter of the aortic valve ring is an essential parameter in the selection of the size of the prosthesis, and the left ventricle outflow tract (LVOT) is less important. The configuration of the CoreValve® prosthesis and implantation technique require that a portion of the valve stent remain lodged in the LVOT (Figure 1). After the implantation of this type of prosthesis, atrioventricular conduction disorders (AVCD) frequently arise, requiring the placement of a permanent pacemaker (approximately 40% of patients).3, 4, 5, 6, 7, 8, 9, 10.
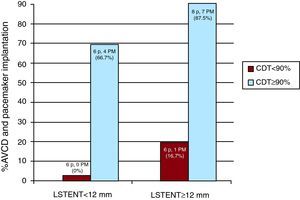
Figure 1. Incidence of atrioventricular conduction disorders and pacemaker implantations based on LSTENT and %CDT. AVCD, atrioventricular conduction disorder; CDT, percentage of the prosthetic stent in contact with the interventricular septum in end diastole; LSTENT, length of the prosthetic stent installed in the left ventricle outflow tract from the aortic ring; PM, pacemaker.
The aim of our study was to detect 2D and 3D transesophageal echocardiography (TOE) parameters associated with the development of AVCD in the 48h following implantation of the CoreValve percutaneous aortic prosthesis, especially considering the relationship of the prosthesis with the basal portion of the interventricular septum (IVS), since this is the area in which the primary vessels can be damaged by the prosthetic stent.1.
MethodsBetween 14 August 2008 and 30 October 2010, CoreValve® percutaneous aortic prostheses were implanted in 35 patients in our hospital (San Carlos Clinical Hospital, Madrid). The hospital's Hemodynamic Department selected the patients and type of prosthesis.10 Patients who already had a pacemaker (n=5) or who died within the first 48h after surgery (n=4) due to causes other than AVCD were excluded from the study, leaving 26 patients for the analysis. Patients signed consent forms to undergo surgery and authorized the use of their data for research purposes.
All patients were monitored during the procedure using Philips iE33 TOE equipment and a Live 3D TOE probe. The images were digitally recorded in the Xcelera system and processed using Qlab software for 3D images (Philips Electronics, Eindhoven, Netherlands). We used 2D, color Doppler, Xplane® biplane, and 3D techniques, choosing the appropriate technique and approach in each individual case for the observation and control of the valve implantation process (aortic valvuloplasty, valve ring-prosthesis alignment, prosthesis deployment, and immediate post-implantation control using 120°-140° views or the Xplane technique at 40°-120°). The following echocardiographic measurements were given special attention: a) length of the prosthetic stent installed in the LVOT from the aortic ring (LSTENT); b) septal contractility prior to implantation, defined as the percentage of IVS incursion into the LVOT in end systole (%IVS); and c) the percentage of the prosthetic stent installed in the LVOT in contact with the IVS in end systole (%CST) and end diastole (%CDT). We measured the penetration and contact zones of the stent (LSTENT, %CST, and %CDT) using 2D TOE in 120°-140° views, offering the best possible view of the LVOT and the prosthetic stent. Using multiple 3D TOE views, we checked for correct placement of the prosthesis immediately following implantation. The %IVS expresses the reduction in LVOT produced by IVS contraction. The %CST and %CDT were obtained by measuring the portion of the stent in contact with the IVS in end systole and end diastole, and are expressed as percentages, with 100% corresponding to complete contact between the IVS and the stent lodged in the LVOT both in systole and diastole. We studied the appearance of AVCD and permanent pacemaker implants within the first 48h after the procedure.
Statistical AnalysisQualitative variables were described as relative frequency, and quantitative variables as mean (standard deviation) or median [interquartile range] in the case of asymmetry. The differences between groups were analyzed using Student t test for quantitative variables and the chi-square test for qualitative variables. We also assessed the interaction between independent variables (%IVS, LSTENT, %CST, and %CDT) to determine if the combined variation of 2 variables modified the studied effect. To evaluate the predictive power of this interaction on AVCD and pacemaker implantation, we categorized quantitative variables by their medians. We performed a multivariate logistic regression in order to detect independent variables in the appearance of AVCD. We analyzed interobserver variability in the measurements made by 2 cardiologists highly experienced in the echocardiographic techniques used, applying kappa indexes. We used SPSS 15.0 (SPSS Inc., Chicago, Illinois, United States) for all statistical analyses.
ResultsMean patient age was 82.8 (6) years, and 15 (57.6%) of them were women. The principal results are summarized in Table 1. The %CDT was associated with the appearance of AVCD after the procedure. There were also correlation trends between AVCD and LSTENT, although these values were not statistically significant. The analysis of interactions between the different independent variables showed an added value for the association of %CDT and LSTENT (Figure 1). There were no AVCD in 6 patients with %CDT<90% and LSTENT<12mm; these did occur in 1 of 6 patients (17%) with %CDT<90% and LSTENT≥12mm, 4 of 6 (67%) with %CDT≥90% and LSTENT<12mm, and 7 of 8 (87.5%) with %CDT≥90% and LSTENT≥12mm (P=.034). In the septal contractility study (%IVS), there was no apparent association with the appearance of AVCD. The multivariate analysis (Table 2) suggested that %CDT was the only independent predictor of AVCD (odds ratio: 1.12; 95% confidence interval, 1.01-1.25; P=.03). The interobserver variability was κ=0.71.
Table 1. Characteristics of the Two Groups According to Implantation or Not of a Pacemaker Following the Procedure.
| No PM | With PM | P | |
| Patients | 14 (53.8%) | 12 (46.1) | |
| Age, year | 83±7.1 | 82.5±4.9 | .930 |
| Women | 8 (57.1) | 7 (58.3) | .820 |
| LVEF, % | 63.9±11.5 | 67.8±10 | .470 |
| Mean gradient | 52.8±12.9 | 46.8±13 | .420 |
| Mitral ring calcium | 14 (100) | 13 (100) | 1 |
| IVS, cm | 1.5±2.8 | 1.6±1.1 | .910 |
| LVOT | 18±1.7 | 18±2.3 | .980 |
| LSTENT, mm | 10.3±5 | 13.5±5.1 | .100 |
| %IVS | 15±9.1 | 13.9±7.3 | .680 |
| %CST | 73±37 | 91.9±27.7 | .140 |
| %CDT | 52±34.5 | 89.2±27.3 | .005 |
| Normal ECG | 12 (85.7) | 7 (58.3) | .210 |
| LBB ECG | 1 (7.1) | 1 (8.3) | .820 |
| RBB ECG | 0 | 1 (7.6) | .520 |
| HMB ECG | 1 (7.1) | 2 (16.7) | .520 |
| IAVB ECG | 0 | 1 (8.3) | .700 |
| EuroSCORE | 18.3±8.3 | 18.6±7.5 | .900 |
| No. 26 CoreValve | 12 (85.7) | 9 (69.2) | .210 |
| No. 29 CoreValve | 2 (14.2) | 3 (25) | .260 |
%CDT, percentage of the prosthetic stent in contact with the IVS in end diastole; %CST, percentage of the prosthetic stent in contact with the IVS in end systole; %IVS, percentage excursion of the IVS into the LVOT in end systole; HBM, hemiblock; IAVB, first-degree atrioventricular block; ECG, electrocardiogram; IVS, interventricular septum; LBB, left branch block; LSTENT, length of the prosthetic stent installed in the LVOT from the aortic ring; LVEF, left ventricle ejection fraction; LVOT, left ventricle outflow tract; PM, pacemaker; RBB, right branch block.
Data are expressed as n (%) or mean ± standard deviation.
Table 2. Multivariate Logistic Regression Analysis for the Study of Predictive Variables for Atrioventricular Conduction Disorders Following the Procedure.
| OR (95% CI) | P | |
| Age | 0.94 (0.74-1.18) | .62 |
| Ejection fraction | 1.06 (0.83-1.35) | .60 |
| Mean gradient | 0.87 (0.70-1.07) | .20 |
| LVOT diameter | 0.59 (0.13-2.67) | .49 |
| Prosthesis number | 0.95 (0.43-2.09) | .90 |
| LSTENT | 1.22 (0.86-1.72) | .20 |
| %CST | 1.46 (0.83-2.56) | .20 |
| %CDT | 1.12 (1.01-1.25) | .03 |
%CDT, percentage of the prosthetic stent in contact with the IVS in end diastole; %CST, percentage of the prosthetic stent in contact with the IVS in end systole; 95% CI, 95% confidence interval; LSTENT, length of the prosthetic stent installed in the LVOT from the aortic ring; LVOT, left ventricle outflow tract; OR, odds ratio.
The appearance of AVCD is a frequent complication4, 5, 6, 9, 10 following the implantation of CoreValve® percutaneous aortic prostheses. The implantation process attempts to align the prosthesis as closely as possible with the native valve ring. Once the prosthesis is deployed, the prosthetic stent penetrates up to 17mm into the LVOT (Figure 2, Figure 3), according to the results obtained in our study. This causes contact between the stent and the underlying IVS to a varying extent. In this region, the distal portion of the bundle of His and the left branch are more superficial and vulnerable to compression.1 Furthermore, these patients usually have hypertrophy of the basal portion of the IVS. The incidence of AVCD and need for pacemaker varies depending on the level of hypertrophy of the basal septum, its contractility, and the size of the prosthesis.4, 5 The echocardiographic analysis shows the contact between the stent and the IVS both in diastole and systole. The extent of contact could explain the incidence of AVCD. In our study, %CDT was the best predictor of AVCD and the need for implanting a pacemaker, better than LSTENT, although the interaction between %CDT and LSTENT was strongly associated with the appearance of AVCD (Figure 1). Septal contractility (%IVS) was not associated with the appearance of AVCD in our study. Based on our data, it appears that the level of contact between the IVS and the stent in diastole is more important. The results from our study could explain the lower number of patients requiring pacemakers after the implantation of an Edwards8 prosthesis, since the penetration into the LVOT and thus the contact with the IVS are lower than with the CoreValve prosthesis. In our study, 3D TOE was a useful technique that facilitated the observation of the prosthesis, its relationship with the native aortic root, and also the configuration of the aortic root. However, it did not provide any advantages over 2D TOE in the measurement of the aortic ring, LVOT, LSTENT, CDS, or CDT, since these measurements cannot be made directly with 3D imaging and require offline analysis.
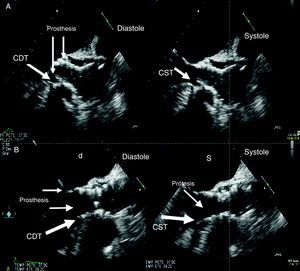
Figure 2. Contact surface between the interventricular septum and the prosthetic stent. A, patient with no contact. B, patient with contact in systole and slight contact in diastole. CDT, contact in diastole; CST, contact in systole.
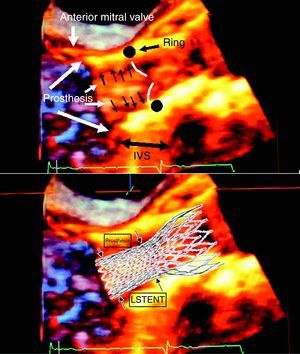
Figure 3. 3D transesophageal echocardiogram of a CoreValve® and diagram of the superimposition on the echocardiographic image. IVS, interventricular septum; LSTENT, protrusion of the prosthetic stent into the left ventricular outflow tract.
The primary limitation in this study was the inability to predict the appearance of AVCD before implanting the valve, since we could not estimate the portion of the stent that would remain in contact with the IVS. Another limitation was the small sample size, although we must keep in mind that this is a rare therapeutic procedure that is still under study. On the other hand, some of the AVCD that appeared in our study might have been resolved if given more time than that established by guidelines for implantation of a permanent pacemaker.
Our results suggest that broad contact between the IVS in diastole and the CoreValve® prosthetic stent, especially when associated with greater stent penetration into the IVS, is correlated with the appearance of AVCD and the need for a permanent pacemaker.
A higher implantation of the prosthesis and smaller stent size could reduce the incidence of these complications, although more studies are needed to confirm this hypothesis.
Conflict of interestsNone declared.
Received 20 February 2011
Accepted 28 August 2011
Corresponding author: Unidad de Imagen Cardiovascular, Servicio de Cardiología, Instituto Cardiovascular, Hospital Clínico San Carlos, Martín Lagos s/n, 28040, Madrid, Spain. calmeria.hcsc@salud.madrid.org