Keywords
INTRODUCTION
Aortic valve stenosis is the most common valvular condition after age 65 (2%-7%), and the prognosis of patients receiving only medical treatment is poor.1 Surgery has shown very good short-term and medium-term results, particularly when there is no formal contraindication for surgery.2 However, many patients present a very high risk due to comorbidity: advanced age, ventricular dysfunction, lung disease, prior surgery, and other conditions. Percutaneous treatment by valvuloplasty was first used in the late 1980s. The immediate results were satisfactory, although obstruction and symptoms recurred within 1 to 6 months.3 At present, valvuloplasty is considered only a bridge to surgery.4 We present the initial experience of percutaneous aortic valve implantation in Spain.
METHODS
Device
The Cribier-Edwards aortic valve consists of a biological valve constructed with equine pericardium fitted over a stainless steel stent, which is mounted onto an expandible balloon for implantation. The valve is available in 2 sizes: 23 and 26 mm.
Patients
Four consecutive patients were included in the REVIVE II research protocol. Candidate requirements were high surgical risk, severe aortic stenosis (valve area £0.7 cm2), and favorable aortic and femoral characteristics based on angiography and computed tomography (CT) study. More information on the inclusion and exclusion criteria for the research protocol is available at the website.
Pre-procedure Studies
Transthoracic and transesophageal echocardiography were used to measure the aortic annulus where the prosthesis was to be implanted. Additionally, coronary angiography was used to assess concomitant coronary disease, and aortography, iliac and femoral arteriography, and CT of the thoracic and abdominal regions were performed to evaluate the feasibility of retrograde access.
Procedure
With the patient under general anesthesia, the femoral artery and vein on one side and the contralateral femoral artery were cannulated. A pacemaker catheter was inserted and tested at 180-200 bpm; the blood pressure drop was confirmed by cancelling heart motion during overpacing. A left and right hemodynamic study was performed before and after the procedure (Figure 1). Preliminary aortography was used to find the view most perpendicular with respect to the valve that would help position the prosthesis correctly. An AL1 catheter and straight hydrophilic guide wire were used to cross the valve and a 300-mm extrastiff Amplatz guide wire of moderate support was introduced. Patients then underwent valvuloplasty with a balloon of 20´30 mm when a 23-mm valve was implanted and 23´30 mm when a 26-mm valve was implanted. Balloon inflation was performed during overpacing and contrast was injected into the aorta to observe relationships between the balloon, valve, and aorta. A technician prepared and fitted the valve with a special device designed for this purpose (Figure 2). The access was then dilated with progressively larger dilators, and a 22 French (Fr) sheath was introduced for the 23-mm valves and a 24 Fr for the 26-mm valves. The catheter carrying the prosthesis was inserted and advanced to the left ventricle, taking advantage of the change in catheter direction achieved with the deflector (Figure 3). Aided by angiography and transesophageal echocardiography, the valve was placed in the correct position; that is, with the proximal end of the prosthesis at the calcium line observed on radioscopy. Once the position was confirmed, the prosthesis was implanted by manually inflating the balloon, while the heart was overpaced to maintain stability. Prosthesis position and functioning were verified by echocardiography, by measuring simultaneous left ventricle-aorta gradients, and by aortography. Lastly, the arterial access was surgically closed in the same interventional cardiology suite.
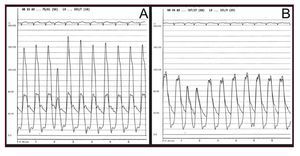
Figure 1. Intraventricular and intraaortic pressure gradients before (A) and after (B) aortic valve implantation.
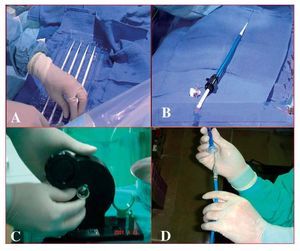
Figure. 2. A: femoral access dilators. B: final arterial introducer. C: mounting of the prosthesis on the balloon. D: prosthesis mounted on the balloon.
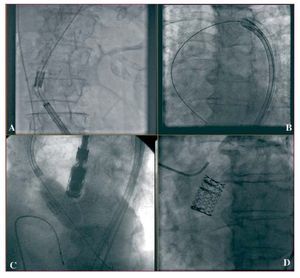
Figure 3. Angiographic sequence of device implantation in the aortic valve. A: catheter introduction through the femoral artery. B: catheter deflection in the aortic arch. C: prosthesis inflation. D: angiographic outcome.
Intravenous heparin was used during the procedure to achieve an activated coagulation time between 250 and 300 s. Loading doses of clopidogrel (300 mg) and acetylsalicylic acid (300 mg) were administered and dual antiplatelet therapy was maintained for at least 6 months.
RESULTS
Patients
Surgery was ruled out in 2 patients because of advanced age (96 and 94 years) and in others because of lung disease. The patients were informed of the techniques and agreed to percutaneous treatment. The clinical and hemodynamic characteristics are shown in Table 1.
Clinical Course
Four patients received the aortic valve replacement without complications and were transferred to the intensive care unit within the first 24 hours. There were no vascular complications. Patient 4 developed acute renal failure (peak plasma creatinine, 2.3 mg/dL; clearance, 24.2 mL/min) that resolved completely in 7 days, at which time creatinine was again at baseline levels (1.3 mg/dL).
The patients were discharged a mean of 4 days after the procedure and have remained clinically stable with improved functional status (NYHA classification II) during follow-up: 4 months (2 patients) and 2 months (2 patients). A follow-up echocardiogram 1 month after the procedure showed that the prosthesis was functioning perfectly in the 4 patients, with no residual gradient. One patient presented moderate aortic regurgitation due to a perivalvular leak. The calculated area was greater than 1.6 cm2 in all cases (Table 2).
DISCUSSION
The incidence of aortic stenosis in patients older than age 65 is estimated at 2% to 9%.5 Following the diagnosis, the gradient increases a mean of 7 mm Hg and the area decreases 0.1 cm2 per year.6 In patients with symptoms, the prognosis is poor with conservative treatment.7 Surgical treatment is the procedure of choice and provides excellent results.8 However, the results are not optimal in patients of very advanced age and those with comorbid conditions.9,10 The valve can be replaced in certain patients of very advanced age with acceptable mortality rates11-13; however, perioperative complications are more common in patients in their 80s. The published results are difficult to extrapolate due to the publication bias of the series with better results and, particularly, because these studies present some bias in the selection of patients for surgery. A study to compare surgery with conservative treatment in 205 elderly patients (age 70 years or older) who were ideal candidates for surgery found low operative mortality (2%).14 Despite this favorable scenario, the authors found no clear benefit and indicated that the surgery would be less advisable in patients in their 80s.15 In these cases, percutaneous implantation could be an alternative. Results of treatment with the Cribier-Edwards prostheses in a series of high-risk surgical patients have recently been published. An intraoperative mortality of 2% and 2-month mortality of 12%, compared with a predicted surgical mortality of 28%, support this new technology.16 Similar to mortality, morbidity, and concomitant complications are also less common with percutaneous aortic valve replacement. Hospitalization can be shortened to a mean of 3 or 4 days, and the main problems of interventional procedures (vascular access, stroke) probably do not exceed 1% or 2%. Other percutaneous aortic valves are also currently used. At present the most extensive experience is probably with the CoreValve (Paris) and the Cribier. The main difference between these devices is that the CoreValve is a longer, self-expanding nitinol prosthesis that covers the ostium of the coronary arteries after implantation, but allows flow through the struts.
The largest series includes 25 patients and the rate of major events during hospitalization was 32% (including the death of 5 [20%] patients).
There are several limitations to percutaneous replacement, in addition to the fact that it is still in the experimental phase. The aortic annulus size is limited because only 2 prosthetic valve sizes are available (in our case it had to be between 18 and 25 mm), and there may be problems in patients with severe peripheral artery disease because of the size of the arterial introducers. The procedure does entail a learning curve, and previous studies have shown a learning curve associated with worse results. Therefore, caution should be exercised in attempting to make the interventional procedure a universal approach. Other limitations, such as massive calcifications that can shift during the procedure or cause significant paravalvular regurgitation, are rare. In any case, these factors are usually related to the novelty of the technique, and their importance will probably diminish in the future. Large studies with long-term follow-up are needed to confirm the initially favorable outcomes achieved with percutaneous aortic valve implantation.
Comment
Since manuscript preparation, 4 additional patients have undergone the procedure successfully and been included in the REVIVE II study protocol. All 8 patients continue to be asymptomatic, with a mean follow-up of 9 to 18 months.
Correspondence: Dr E. García.
Sección de Hemodinámica. Servicio de Cardiología. Hospital Universitario Gregorio Marañón.
Dr. Esquerdo, 46. 28007 Madrid. España.
E-mail: ejgarcia@retemail.es
Received March 14, 2008.
Accepted for publication August 22, 2008.