Keywords
Received January 28, 2005. Accepted for publication April 29, 2005.
INTRODUCTION
The mitral-aortic fibrosa is a thin layer of fibrous tissue that separates the posterior portion of the aortic root from the base of the anterior mitral leaflet. Its posterior and lateral limit is defined by the left atrium, the inferior limit by the left ventricular outflow tract (LVOT), and the upper limit by the pericardium.1-4 Pseudoaneurysm of the mitral-aortic fibrosa (PMAF) or intervalvular fibrosa is an uncommon condition, consisting of a pulsatile cavity in the mitral-aortic junction communicating with the left ventricular outflow tract. The most frequent cause is aortic valve endocarditis, particularly in prosthetic valves.1-2 We present a case of asymptomatic PMAF developing late following aortic valve replacement surgery and treated by percutaneous closure.
CLINICAL CASE
A 69-year-old woman with a history of hypertension presented syncope on exercise. She was diagnosed with severe degenerative aortic stenosis, with a peak gradient of 130 mm Hg and a mean of 81 mm Hg. The mitral valve and ventricular function were normal and coronary angiography showed normal coronary vasculature. The patient underwent surgery with implantation of a Toronto SPV® size 23 aortic stentless bioprosthesis; there were no postoperative complications. Follow-up echocardiography showed no relevant abnormalities.
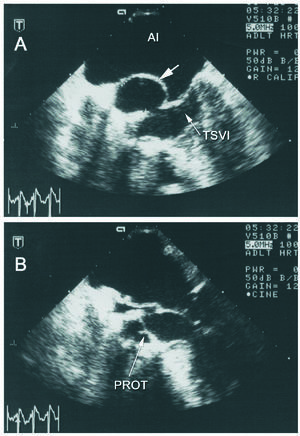
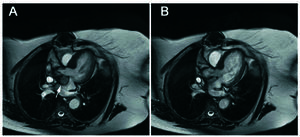
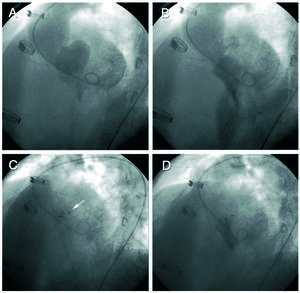
Seven years following the procedure the patient remained asymptomatic. On transthoracic echocardiography (TTE) the aortic bioprosthesis continued functioning normally and an anechoic pulsatile space protruding toward the left atrium was observed in the region of the aortic valve. Transesophageal echocardiography (TEE) disclosed a 30×25 mm pulsatile cavity in the mitral-aortic junction, communicating with the left ventricular outflow tract (LVOT) by a 1-cm neck, with systolic expansion toward the left atrium and diastolic collapse (Figure 1). Cardiac magnetic resonance cardiography (Figure 2) confirmed the findings and established the diagnosis of pseudoaneurysm of the mitral-aortic intervalvular fibrosa. In view of the surgical risk and to prevent complications, percutaneous treatment was decided. The procedure was performed under TEE and angiographic monitoring (Figure 3), the latter using a 6 French pigtail catheter in the LVOT inserted by a right femoral approach. A 260-cm Radiofocus wire (Terumo Corporation, Japan) was inserted in the left brachial artery and advanced to the interior of the PMAF, and with the help of a multipurpose catheter, was exchanged for an Amplatzer® wire. A 12-mm Amplatzer® Muscular VSD Occluder device (AGA Medical, USA) was advanced over the wire and placed in the neck of the aneurysm (the diameter of the device chosen was slightly larger then the neck). Positioning of the device was confirmed by ventriculography in two views (RAO 30° and LAO 45°) and by TEE, to assure that there was no interference with the aortic bioprosthesis or mitral valve function. After deployment of the device, TEE and angiography showed absence of flow to the cavity. Transesophageal echocardiography performed at 6 months confirmed these findings and the patient remained asymptomatic at 1 year of follow-up.
Figure 1. Transesophageal echocardiogram; transverse view in the left ventricular outflow tract (LVOT). A: image in systole showing the pseudoaneurysm (arrow) protruding toward the left atrium. The orifice of the communication with the LVOT is evident. B: image in diastole showing collapse of the pseudoaneurysm. The normally functioning aortic bioprosthesis (PROT) can be seen in both images. LA indicates left atrium.
Figure 2. Magnetic resonance. Transverse slice. The images in systole (A) and diastole (B) depict the pulsatile movement of the pseudoaneurysm, which is displaced in systole toward the left atrium (arrow).
Figure 3. Left ventriculography in the left anterior oblique view. The upper images show expansion of the pseudoaneurysm in systole (A) and collapse in diastole (B). The lower images show the Amplatzer device (arrow) after deployment (C) and the final ventriculography, in which there is no passage of contrast toward the cavity (D).
DISCUSSION
Pseudoaneurysm of the mitral-aortic fibrosa usually occurs in patients with a history of aortic valve replacement due to endocarditis. It is more commonly associated with prosthetic valves, probably due to abscess formation at the ring, which fistulizes toward the outflow tract.1-4 There are also reports of cases, such as the one described, occurring after aortic valve surgery for processes other than endocarditis.5 Partial dehiscence of an aortic prosthesis can contribute to the formation of PMAF.3 Sometimes, as in our case, the pseudoaneurysm is diagnosed years after the surgical intervention.4-6 Isolated cases secondary to chest trauma have also been described.7
The clinical spectrum varies from asymptomatic cases to cardiac tamponade due to rupture of the PMAF toward the pericardial cavity.8 Progressive enlargement of the pseudoaneurysm can produce angina due to compression of the coronary arteries,6,9,10 and fistula may form toward the anterior chest wall.4 Heart failure is another manifestation, occurring when the pseudoaneurysm communicates with the ascending aorta or left ventricle; the clinical picture is similar to that of aortic or mitral regurgitation, respectively. Severe mitral regurgitation caused by displacement of the anterior mitral leaflet has been described.11 Another manifestation is stroke, occurring as a consequence of embolization of the thrombotic material within the PMAF.12
The recommended treatment for PMAF is surgery even in asymptomatic patients,2 in order to prevent the development of complications. Resection and repair of the defect4,10 can be done with or without aortic valve replacement5,6,10 or homograft replacement of the aortic root.2 When there is coronary involvement, a bypass may be necessary.6,9 Surgery for this condition is technically complex and patients often have a background of prior surgery; hence the associated morbidity and mortality is not negligible.
To the best of our knowledge, the case presented is the first description of percutaneous treatment for PMAF. The rationale for this approach was that isolation of the cavity from the LVOT would prevent enlargement of the pseudoaneurysm and potential complications. We conclude that this is a viable technique that can be performed safely, yields good results at middle term, and may be an alternative to surgical treatment.
Correspondence: Dr. S. Jiménez Valero.
Unidad de Hemodinámica. Hospital de Madrid Montepríncipe.
Avda. Montepríncipe, 25. 28660 Boadilla del Monte. Madrid. España.
E-mail: sjvcardio@yahoo.es