To the Editor,
Paravalvular leaks (PVL) develop after implantation of prosthetic heart valves in 2% to 12% of the cases, and the standard treatment is repeat valve replacement.1 Nevertheless, although the subject is controversial,2 it has recently been shown that it is feasible to apply percutaneous closure in a growing number of patients.3, 4, 5 The percutaneous closure of mitral PVL is more complex than that of aortic leaks since it is necessary to establish a circuit with a long guide wire along which the delivery sheath is advanced.3, 4, 5 In patients with mitral PVL who also have a metallic aortic valve prosthesis, this device adds to the difficulty because the guide wire can interfere with the aortic prosthesis and complicate the procedure. For this reason, some surgeons and interventionalists opt for the transapical approach.6
In our center, percutaneous closure of a mitral PVL has been successfully performed in 2 patients who also had metallic aortic valve prostheses. The first case was that of a 79-year-old woman with heart failure in New York Heart Association functional class IV, who had undergone valve surgery 18 years earlier. She had a normally functioning 21-mm Björk prosthetic valve in aortic position and a 27-mm Björk prosthetic valve in mitral position, with posterior dehiscence that produced severe periprosthetic regurgitation. In addition, she had severe left ventricular dysfunction, chronic atrial fibrillation, and a history of stroke with sequelae, and had undergone placement of an implantable cardioverter defibrillator. The estimated surgical risk was 17% according to the EuroSCORE and 18% according to the Society of Thoracic Surgeons score. In this patient, percutaneous closure of the PVL was carried out successfully and without complications; it involved the implantation, in 2 separate procedures, of a 14/5-mm and an 8/3-mm Amplatzer Vascular Plus III occluder, respectively.
The second patient was a 74-year-old woman who, 26 years earlier, had undergone the implantation of a 21-mm Omnicarbon aortic valve prosthesis, which was functioning normally, and a 27-mm Omnicarbon mitral valve prosthesis with posterior dehiscence and moderate regurgitation. She had moderate heart failure (New York Heart Association functional class II) and severe hemolysis requiring frequent blood transfusions. She also had a history of stroke with sequelae and presented with atrial fibrillation. Her EuroSCORE was 13% and her Society of Thoracic Surgeons score, 7%. Percutaneous closure with a 10/3-mm Amplatzer Vascular Plug III occluder (AGA Medical) was carried out successfully and without complications.
In both patients, the procedure was performed under general anesthesia using 3-dimensional transesophageal echocardiography (Figure). Transseptal puncture was carried out using the standard technique (Müllins sheath and Brockenbrough needle). Once access to the left atrium had been gained, a 6-Fr angiography catheter was introduced to enable access to the left ventricle using a hydrophilic guide wire. After crossing the dehiscence, the guide wire was advanced through the aortic valve prosthesis to the descending aorta where, following retrograde mobilization of the loop system from the femoral artery, it was entrapped and externalized through the femoral artery. Once this circuit had been established, the delivery sheath was advanced, together with its dilator, through the interatrial septum, the PVL, and left ventricle.
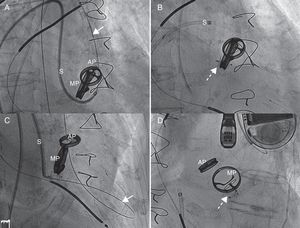
Figure. Implantation of two devices in separate procedures. A: guide wire (arrow) crossing adjacent to the mitral valve prosthesis, through the dehiscence, with the sheath crossing over into left ventricle. B: image following implantation of the first device (broken arrow). C: implantation of a second device, with the guide wire (arrow) crossing adjacent to the first device. D: completion of the second procedure; image showing the two correctly placed devices (broken arrow). AP, aortic prosthesis; MP, mitral prosthesis; S, sheath.
The Vascular Plug III occluder was then advanced to left ventricle, where the ventricular portion was released and, after it had been withdrawn to the level of the annulus of the mitral valve prosthesis, was released in its entirety, and correctly placed in all 3 procedures.
Both cases involved certain difficulties, in addition to those customarily encountered, derived from the presence of the metallic aortic valve prosthesis: a) the initial advance of the guide wire had to be anterograde (from atrium to ventricle), against the flow through the PVL, a circumstance that made it more difficult to advance the guide wire; b) once the guide wire had passed through the aortic valve prosthesis, it interfered with the movement of the valve, a situation that provoked moments of hemodynamic deterioration that required the mobilization of the guide wire in order to restore the movement of the prosthesis; for this reason, it is important to maintain continuous monitoring with the aid of echocardiography, fluoroscopy, and hemodynamic data; c) in patients who do not have a metallic aortic valve prosthesis, once the sheath is advanced into the ventricle, the circuit involving the guide wire is often maintained to facilitate the advance of the device, minimize technical complications should the sheath be withdrawn, and enable the implantation of a second device during the same procedure, should it be necessary; however, due to the problems resulting from the blockage of the aortic valve prosthesis, in these cases, once the sheath had been advanced into the ventricle, the guide wire was withdrawn in order to reduce the time during which the aortic valve prosthesis would be susceptible to potential blockage; and d) lastly, if, as a result of the aforementioned circumstances, it should be necessary to place 2 devices in order to achieve complete repair of the dehiscence, they should be implanted in separate procedures, as in our first patient.
Despite these difficulties, the cases we report show that percutaneous closure is a feasible treatment option in patients with mitral PVL who also have metallic aortic prostheses.
.
Corresponding author: raulmorenog@terra.es