Percutaneous treatment of stenotic lesions in the pulmonary artery system has been shown to be a valid and effective intervention, in both adults and children.1,2 The development of an iatrogenic aortopulmonary fistula after pulmonary angioplasty is a rare complication described by several authors who have chosen different devices for percutaneous closure of the lesion.3–6 The present letter discusses this uncommon lesion and considers the percutaneous treatment options.
A 13-day-old boy diagnosed with transposition of the great arteries underwent an arterial switch operation and a Lecompte maneuver. In the operating room, he was diagnosed with a coronary anatomy consisting of an intramural left coronary artery, from which the left anterior descending artery and the right coronary artery originated. There was an independent filiform circumflex artery. To correct the defect, a 3.5-mm PTFE tube was placed between the ascending aorta and the left coronary artery. The postoperative outcome was satisfactory, and the patient was discharged 14 days after the procedure.
In subsequent follow-up, the patient was found to have supravalvular pulmonary stenosis with an echocardiographic gradient of 62mmHg. A cardiac catheterization procedure was performed when the patient was 1 year old. A stenosis gradient of 52mmHg was detected, along with right ventricular pressures that were 66% of systemic pressures and stenosis at the origin of the right pulmonary artery (gradient, 18mmHg). Percutaneous angioplasty of the pulmonary artery and origin of the right pulmonary artery was performed with a balloon catheter measuring 15×30mm and 10×20mm, respectively, using an 8-Fr introducer sheath. Disappearance of the gradient in the branch was confirmed and the gradient in the main artery decreased to 16mmHg. There was also a mild tear in the intimal layer at the origin of the right pulmonary artery. However, magnetic resonance imaging showed the integrity of the wall of the main artery and branch.
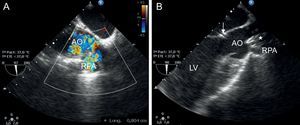
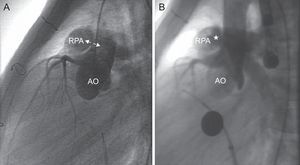
The patient was asymptomatic for the next 4 years until signs of congestive heart failure developed. Echocardiography revealed a progressive increase in the size of the left heart chambers, along with continuous flow at the origin of the right pulmonary branch and retrograde flow in the descending aorta. Because an aortopulmonary window secondary to angioplasty was suspected when the patient was 5.5 years old and weighed 22kg, cardiac catheterization and transesophageal echocardiography were performed. An 8-mm fistula was observed between the ascending aorta and the origin of the right pulmonary artery. The mean aortic and pulmonary pressures were 65mmHg and 28mmHg, respectively, with a QP/QS ratio of 2.3 and a suprapulmonary gradient of 15mmHg and 8mmHg at the origin of the right pulmonary branch. The atrial fistula was closed in a percutaneous procedure using a 9-mm Amplatzer® Septal Occluder device, chosen in view of the size of the lesion. The defect was probed from an aortic approach with a 4-Fr distal needle catheter and 0.014” hydrophilic guidewire. Once in the pulmonary lumen, an arteriovenous loop was created by capturing the guidewire with a 10-mm loop catheter, allowing the guidewire to leave via the femoral vein. Once the guidewire was in place, the distal needle catheter was advanced to place a more supportive guidewire (0.035”), over which the sheaf was placed (Amplatzer® 7 Fr Delivery System) and the device was deployed using the pulmonary approach. The procedure was a success; there were no incidents or need for repositioning (Figure 1 and Figure 2). After closure, a residual shunt through the device was observed (QP/QS = 1.5), without increasing either the pulmonary gradient or affecting the ascending aorta. The shunt could be detected in echocardiographic studies for 1 month. In addition, there was mild hemolysis that disappeared 6 days after the intervention without requiring any treatment. The patient was asymptomatic on the 5th day after the procedure. After 28 months of follow-up, there have been no new complications.
Transesophageal echocardiography. A: Measurement of the aortopulmonary window. B: Check for correct deployment of the device (asterisk) without any change in aortic valve function or outflow of the coronary conduct (arrow). AO, aorta; RPA, right pulmonary artery; LV, left ventricle.
Cardiac catheterization. Lateral aortography. A: Visualization of the window (arrows) between the posterior wall of the main pulmonary artery and right pulmonary artery and anterior wall of the aorta. B: Check for correct deployment of the device (asterisk) without affecting coronary perfusion, with residual shunt through the device. AO, aorta; RPA, right pulmonary artery.
Percutaneous treatment of stenotic lesions of the pulmonary artery branch has been accepted as an effective and valid option.1,2 There are isolated reports of patients with an iatrogenic shunt between the aorta and the pulmonary artery, especially in patients who have undergone an arterial switch, as was the case in our patient.3–6 The etiopathogenesis has been attributed to the widely reported adherence between the aorta and pulmonary artery on performing the Lecompte maneuver in arterial switching.5
The progressive development of symptoms of heart failure due to the iatrogenic window is due to the progressive increase in the size of the lesion, as explained by Vida et al4 and as occurred in our patient.
When percutaneous closure is performed, care should be taken when choosing the device so as not to affect normal pulmonary valve function, to ensure sufficient coronary perfusion, and to keep obstruction of the lumen of both arteries to a minimum. Drug-eluting stents have been used during the closure procedure.3,4 These are indicated in lesions close to pulmonary branching, as another device would lead to protrusion into the lumen of the pulmonary arteries. Amplatzer® Duct Occluder II devices and Amplatzer® Septal Occluder devices have also been used,6 above all for small lesions. The reason for our choice was the better profile that adapts to the vascular lumen without occluding the lumen of the 2 vessels and the outflow of the conduct that irrigates the coronary arteries.