The therapeutic positioning report (TPR) that regulates the funding of direct oral anticoagulants (DOACs) for patients with nonvalvular atrial fibrillation dates from 2016 and has not been updated with newer information from clinical trials1 and clinical practice registries.2
In addition, there are cost analyses from a social3 and clinical perspective that include hemorrhagic and thromboembolic complications.4 However, there is no comparative analysis of the care burden of vitamin K antagonists (VKAs) vs DAOCs. Therefore, we designed a study to analyze the care burden and economic costs in patients who were switched from a VKA to a DOAC, comparing the 6 months before and 6 months after starting the DOAC.
We performed a retrospective, observational study to evaluate the quality of care in the Noia Primary Care Service (PCS) of the Santiago de Compostela and Barbanza health care area. The Noia PCS, with 24 primary care physicians (PCPs) and 25 primary care nurses (PCNs), provides coverage to a population of 32 196, of whom 51.3% are women and 29.4% are older than 65 years. The study received the approval of the Galicia ethics committee for research involving medicines and was carried out following a current data management protocol.
Currently, after completing a guided training program for PCPs and PCNs working in health centers, the PCNs measure the international normalized ratio (INR) using portable coagulometers. The PCNs also adjust the VKA dose using a centralized computer program, unless the patient's INR is <1.8 or> 4.2, or they have a metal heart valve, in which case this is done by the PCP.
The data set was generated during the prescription authorization process using the electronic medical record program IANUS and the pharmacy database SIAC-PF. The data set was regularized in February 2020 in accordance with Directive 7/2019 of the Galician Regional Government Department of Health on the protocol for the management of data supplementary to medical records.
We included all patients with nonvalvular atrial fibrillation who were switched from a VKA to a DOAC between September 2015 and September 2019 and who met the following criteria: a) on treatment with a VKA for at least 7 months before the switch and on a DAOC for 6 months after the switch; b) all the switches from VKA to DAOC were made in line with the criteria and general recommendations of the TPR, since, to authorize starting a DOAC, the pharmacy inspector must review the patient's medical record.
The primary outcome variable was the number of PCP, PCN and hospital consultations, with costs calculated according to those published in Decree 56/2014 of the Galician Regional Government, which establishes the rates for health care services provided in health centers of the Galician Health Service.5 For hospital consultations, the cost was calculated using a weighted method between first, follow-up, and high-resolution or one-stop consultations. Secondary outcome variables were the costs of low molecular-weight heparin (LMWH), oral anticoagulants (VKAs and DAOCs), INR test strips, and the follow-up monitoring blood tests performed.
To estimate the cost of test strips, we used the results of the ANFAGAL+ study, in which a mean 10.7±0.2 measurements were performed over an observation period of 7.6±0.2 months,5 entailing a mean expenditure of €2.70 in 6 months.6 The expenditure on blood tests was estimated using the cost provided by our central laboratory, based on an annual analysis for all patients (€5.33/y) except those older than 75 years or with a glomerular filtration rate <60mL/min, who require twice-yearly analyses (€10.66 /y).6
The variables analyzed, which were all quantitative and with nonnormal distribution, are presented as median [interquartile range] and were compared against each other using the Wilcoxon test. The statistical package SPSS 24.0 for Windows was used, and results with P <.05 were considered statistically significant.
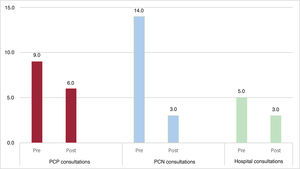
A total of 202 patients were included, of whom 50% were women, with a median age of 82 [74-86] years. After the switch to DOACs, there was a decrease in all consultations: 2.5 PCP consultations/patient (33.3%), 10.0 PCN consultations/patient (75%) and 2.0 hospital consultations/patient (37.5%) (table 1, figure 1).
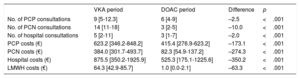
Primary care and hospital consultations in the 2 periods and associated costs
| VKA period | DOAC period | Difference | p | |
|---|---|---|---|---|
| No. of PCP consultations | 9 [5-12.3] | 6 [4-9] | –2.5 | <.001 |
| No. of PCN consultations | 14 [11-18] | 3 [2-5] | –10.0 | <.001 |
| No. of hospital consultations | 5 [2-11] | 3 [1-7] | –2.0 | <.001 |
| PCP costs (€) | 623.2 [346.2-848.2] | 415.4 [276.9-623.2] | –173.1 | <.001 |
| PCN costs (€) | 384.0 [301.7-493.7] | 82.3 [54.9-137.2] | –274.3 | <.001 |
| Hospital costs (€) | 875.5 [350.2-1925.9] | 525.3 [175.1-1225.6] | –350.2 | <.001 |
| LMWH costs (€) | 64.3 [42.9-85.7] | 1.0 [0.0-2.1] | –63.3 | <.001 |
VKA, vitamin K antagonist; DOAC, direct oral anticoagulant; PCP, primary care physician; PCN, primary care nurse; LMWH, low molecular-weight heparin.
Values are expressed as median [interquartile range].
Changes in the number of primary care and hospital consultations in the two analysis periods. PCN, primary care nurse; Post, period with direct oral anticoagulants (DOACs) after changing the type of oral anticoagulant; Pre, period on vitamin K antagonists (VKAs) before changing the type of oral anticoagulant.
The analysis of drug-associated costs showed a significant decrease, of €63.3 (95% confidence interval [95% CI], −84.8 to −41.8; P < .001), in the use of LMWH.
The final balance showed that the introduction of DOACs entailed a decrease of €265.7 (−€997.1 to €258.7) per patient over 6 months. The reduction in costs for the whole of our PCC had a total saving of €89 654, 40.1% of the cost incurred in the period of treatment with VKA.
Regarding the external validity of this study, the interpretation of these results should be limited to patients who are followed up as outpatients, in a clinical management setting that has access to a shared electronic medical record, a tool that improves communication between care levels and facilitates continuity of care and comprehensive patient care.
In summary, we can conclude that, after switching from VKA to DOAC in line with the 2016 TPR for PCC outpatients, there was a reduction in care burden at all levels of care. The overall financial balance for the PCC, including the costs of oral anticoagulants, the INR test strips, and blood tests, had a saving of nearly €90 000, representing a 40.1% reduction in costs.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSA. Pia-Morandeira conceived the idea and curated the database. All the authors participated in the writing, review, and approval of the manuscript.
CONFLICTS OF INTERESTS. Cinza-Sanjurjo has received funding for a research study from Bayer Hispania S.A. and honoraria for consultation and presentations from Bayer Hispania S.A., Boehringer Ingelheim, and BMS-Pfizer. The other authors declare no conflicts of interests.