Keywords
INTRODUCTION
Cardiomyopathy secondary to iron deposition in thalassemia major is the main cause of death in patients with this condition.1,2 Early identification of the tissue iron deposits is important, particularly in the cardiac tissues, so that chelating therapy can be initiated while the process is still reversible. Magnetic resonance imaging (MRI) is a useful technique for this purpose, since it can detect and quantitatively estimate the amount of iron deposited in various organs.3-5 Recent studies have shown that combined deferoxamine and deferiprone treatment is effective when heart failure develops despite standard chelating therapy with deferoxamine.
CASE STUDY
A 22-year-old male with homozygous beta-thalassemia (beta/delta beta) under treatment with transfusion support every 3 weeks and subcutaneous deferoxamine since the age of 14 was hospitalized for symptoms of progressive dyspnea and edema of the lower limbs. Transthoracic echocardiography showed biventricular dilated cardiomyopathy with severely affected left ventricular systolic function and an ejection fraction (EF) of 20%. Serum ferritin at the time of hospital admission was 1645 ng/mL and the analyses showed liver function alterations (GOT, 84 U/L; GPT, 110 U/L; alkaline phosphatase, 467 U/L; LDH, 687 U/L; and gamma-GPT 36 U/L).
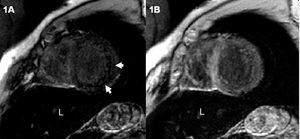
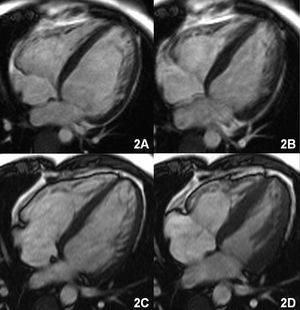
Magnetic resonance imaging was performed to visualize the cardiac hemochromatosis. The gradient echo sequences (FLASH, TR/TE 700/11, flip angle 30°) showed an overall decrease in the myocardial signal that was more pronounced in the posterior and lateral segments, and a notably decreased liver signal (Figure 1A). The functional sequences confirmed volume increases in both ventricles with severely depressed biventricular function (left-ventricular end-diastolic volume [LVEDV] and left ventricular end-systolic volume [LVESV] of 130 and 87 mL/m², respectively, with an EF of 32%) (Figure 2A and B).
Figure 1. A: short-axis, gradient-echo imaging sequence shows a decreased myocardial signal that is more pronounced in the posterior and lateral segments (arrows) and in the liver (L). B: short-axis gradient-echo sequence after treatment shows normal myocardial signal and persistently hypointense liver signal.
Symptomatic treatment was established with diuretics, captopril, and digoxin, and the standard chelating treatment with deferoxamine was changed to combined deferoxamine plus deferiprone according to the following weekly dosing regimen: oral deferiprone 75 mg/kg/day for 4 days and subcutaneous deferoxamine 3 g/day + oral deferiprone 50 mg/kg/day for the remaining 3 days.
The patient's symptoms gradually improved. Six months after starting treatment serum ferritin values were 331 ng/mL and liver function had improved (GOT, 35 U/L; GPT, 46 U/L; alkaline phosphatase, 119 U/L; LDH, 541 U/L; and gamma-GPT 28 U/L). The MRI study one year later showed a marked reduction in ventricular volumes and a notable improvement in biventricular function (LVEDV, 100 mL/m²; LVESV, 50 mL/m²; EF, 52%) (Figure 2C and D). The myocardial signal had normalized on the gradient echo imaging sequences, although a markedly hypointense signal persisted in the liver (Figure 1B).
Figure 2. A (diastole) and B (systole): cine magnetic resonance sequence in four-chamber view shows increased volume of both ventricles with involvement of systolic function. C (diastole) and D (systole): volume reduction and improved systolic function following treatment.
DISCUSSION
Magnetic resonance detection of iron deposition in the organs is based on the effect produced by ferritin and hemosiderin on the tissue proton relaxation time, which becomes shorter. This results in a decrease in the intensity of the signal (darker areas), particularly in T2-weighted sequences.6 Gradient echo sequences are especially sensitive for this purpose and are able to detect even a slight overload of tissue iron. Although there is a correlation between the signal decrease (particularly in T2* sequences) and the degree of iron overload, at the present time there is no accepted standard method to quantify iron overload in the organs, particularly in the heart, because of the difficulty to obtain biopsy specimens for the correlation.7
Deferiprone is a molecule 5 times smaller and more lipophilic than deferoxamine, allowing better intracellular penetration. Once it is bound to iron, it becomes a more hydrophilic complex that is easily transported out of the cell and eliminated. Deferiprone has been shown to be more effective than deferoxamine, particularly for the elimination of myocardial iron.4,8 Moreover, combined therapy (deferiprone + deferoxamine) results in greater chelating activity and therefore, better results than deferoxamine alone, with good tolerance and no reports of major side effects.9
Thus, MRI is a useful diagnostic imaging method for early detection of cardiac hemochromatosis, provides the possibility to establish prophylactic chelating therapy, and allows monitoring of the treatment response once cardiac dysfunction has occurred. A combined treatment regimen with deferoxamine and deferiprone should be assessed in cases in which intravenous treatment with deferoxamine alone is not effective.
Correspondence: Dr. J. Estornell Erill.
Unidad de Tomografía Computarizada y Resonancia Magnética.
ERESA. Hospital General Universitario.
Avda Tres Cruces, s/n. 46014 Valencia. España.
E-mail: jestornell@eresa.com
Received March 23, 2005.
Accepted for publication June 8, 2005.