Keywords
INTRODUCTION
Subaortic stenosis (SAS) occurring late after surgical repair of an atrial septal defect (ASD) is uncommon. The diagnosis is usually made 6 to 8 years after correction of the ASD in childhood. We present the case of a patient who underwent surgery as an adult and was diagnosed with severe SAS 22 years later.
CASE STUDY
A 69-year-old woman who underwent surgery at the age of 48 for ostium primum ASD was seen for dyspnea on moderate exertion. The defect had been surgically closed with a Teflon patch and suturing was used to correct the cleft in the anterior leaflet of the mitral valve. The patient required implantation of a VVI pacemaker to treat a complete atrioventricular block. She remained asymptomatic up to 22 years later, at which time she presented with non-specific chest pain. A stress test had to be interrupted because of dyspnea. Echocardiography showed a hypertrophied left ventricle with preserved function and severe pulmonary hypertension. There were signs of mitral valve fibrosis with moderate regurgitation, and mild aortic regurgitation. A constant systolic gradient of 84 mm Hg was seen in the left ventricular outflow tract (LVOT), caused by a membrane that seemed to originate at the anterior mitral valve. Severe stenosis of the left coronary trunk was diagnosed on cardiac catheterization.
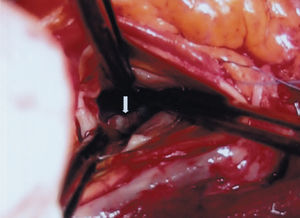
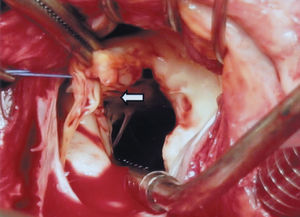
During the intervention, a fibrous protrusion of tissue was found at 2 mm from the aortic valve (Figure 1) and a fibromuscular tract was seen in the septal portion, extending to the mitral-aortic continuation, where a mitral papillary muscle was found to be abnormally inserted (Figure 2). The fibrous protrusion and part of the underlying muscle tissue was resected, the anomalous papillary insertion was sectioned, the destructured mitral valve was replaced by a mechanical prosthesis, and coronary revascularization was performed. The patient presented no postoperative incidents and the residual LVOT gradient was 16 mm Hg on echocardiography.
Figure 1. Fibromuscular protrusion of tissue in the left ventricular outflow tract, 2 mm below the aortic valve ring.
Figure 2. Anomalous insertion of the chordae tendineae of the mitral papillary muscle in the upper portion of the septum, contributing to stenosis of the left ventricular outflow tract.
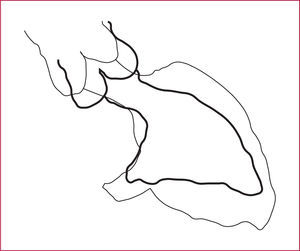
In the review of angiographic findings obtained before the first surgery, there was no LVOT gradient, but a "gooseneck" systolic-diastolic deformity was evident (Figure 3). The echocardiogram showed some hypoplasia of the left chambers and apparent insertion of chordae tendineae in the upper portion of the septum.
Figure 3. Schematic representation of the ventriculography findings before the first procedure, showing the ventricle in systole and diastole. The "gooseneck" image and left ventricular outflow tract deformity remain in systole. The ratio between the length of the left ventricular outflow and the left ventricular inflow tracts is <1.
DISCUSSION
Subaortic stenosis following corrective surgery for ostium primum ASD is uncommon. The condition affects 2% to 15% of patients and is usually detected 6 to 8 years after repair of the defect in childhood.1-4 Few studies include postoperative follow-up of adults,1,4,5 in whom the evolution is slower. The incidence of SAS in these patients may be underestimated, since (as occurred in the case presented) 60% to 70% remain silent for years.3
Subaortic stenosis can be acquired and progressive, and can recur after surgical treatment.6,7 The etiology of this entity is multifactorial. Morphological features and possibly genetic factors8 may predispose the patient to develop SAS. Acquired SAS is associated with fibrous hyperplasia secondary to the development of turbulent flow in the LVOT. Closure of the mitral cleft with narrowing of the outflow tract, which limits systolic displacement of the valves far from the interventricular septum, contributes to the turbulent flow.3 Progression of the condition leads to left ventricular and septal hypertrophy, which is added to the initial LVOT stenosis.6 As in the case presented, a ratio of less than 0.7 between the mitral valve-to-apex distance (left ventricular inflow tract) and the aortic valve-to-apex distance (LVOT) (with normal conditions being 1) favors the appearance of SAS on follow-up.9,10 This characteristic results in the angiographic "gooseneck" morphology in ventricular diastole. The persistence of this sign in systole (as in our case) is considered a predisposing factor for later development of SAS. On echocardiography, this feature is seen as a reduction in the angle formed between the LVOT and the interventricular septum,10 which, being associated with inferior displacement of the insertion plane of the atrioventricular valves toward the left ventricle, predicts a risk of later development of SAS. Although this is also the causal mechanism for congenital SAS, the late diagnosis is less frequently due to anomalous insertion of the papillary muscle in the upper portion of the septum.2,6
CONCLUSIONS
Whenever there are imaging signs of this special morphology within the spectrum of incomplete atrial septum defects, and even though SAS has been excluded intraoperatively, echocardiographic monitoring should be performed periodically to rule out the development of this entity, since a high percentage of patients remain asymptomatic.
Correspondence: Dra. Y. Carrascal.
Servicio de Cirugía Cardíaca. Hospital Universitario de Valladolid.
Avda. Ramón y Cajal, s/n. 47003 Valladolid. España.
E-mail: aguerrerop@medynet.com
Received March 11, 2005.
Accepted for publication May 26, 2005.