Pulmonary hypertension (PH) in patients with heart failure and reduced ejection fraction is associated with a worse prognosis and increases the risk of complications and death in heart transplant (HTx) patients.1 International HTx guidelines thus consider PH to be a relative contraindication for this procedure.2 PH is defined by systolic pulmonary artery pressure >50mmHg, transpulmonary gradient >12mmHg, pulmonary vascular resistance >3Wood units despite optimal medical therapy, and irreversibility following a vasodilator challenge.
The European heart failure guidelines3 recommend left ventricular assist device (LVAD) implantation as a means of reducing PH and providing a bridge to HTx candidacy for patients with irreversible PH.4 Despite not being recommended by the European PH guidelines,5 pulmonary vasodilators, and sildenafil in particular, are also used either alone or in combination with LVADs in specialized HTx centers.6
To evaluate the effect of pulmonary vasodilators and LVAD implantation in HTx candidates with severe PH, we retrospectively reviewed the clinical outcomes of all such patients who underwent vasodilator testing at our hospital between January 2010 and August 2018. The primary objective was to analyze survival outcomes at 2 years following an initial vasodilator challenge.
Patients who met the reversibility criteria during the first vasodilator challenge were classified as eligible for HTx and added to the waiting list; those with a negative challenge were treated with sildenafil and/or LVAD implantation. They were administered a second challenge after 3 to 4 months and added to the HTx waiting list if they met the reversibility criteria. The patients were thus divided into 3 groups: a) patients with reversible PH after the first vasodilator challenge, b) patients with irreversible HTP treated with centrifugal LVAD implantation (HVAD [Medtronic] or HeartMate 3 [Abbott]) (if they received this treatment before HTx), and c) patients with irreversible PH not treated with centrifugal LVAD implantation (ie, those who received a pulsatile LVAD (EXCOR [Berlin Heart] or sildenafil monotherapy).
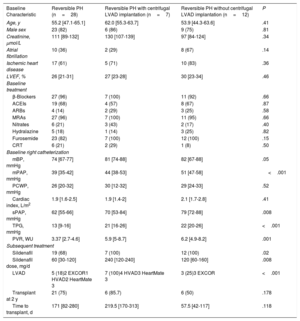
Patient characteristics and right heart catheterization data at baseline are summarized in table 1, together with details of the treatments received. Quantitative variables are presented as median [interquartile range] and dichotomous variables as number and percentage. Between-group differences were calculated using the Kruskal-Wallis test for quantitative variables and the chi-square test for dichotomous variables.
Baseline characteristics and subsequent treatments
| Baseline Characteristic | Reversible PH (n=28) | Reversible PH with centrifugal LVAD implantation (n=7) | Reversible PH without centrifugal LVAD implantation (n=12) | P |
|---|---|---|---|---|
| Age, y | 55.2 [47.1-65.1] | 62.0 [55.3-63.7] | 53.9 [44.3-63.6] | .41 |
| Male sex | 23 (82) | 6 (86) | 9 (75) | .81 |
| Creatinine, μmol/L | 111 [89-132] | 130 [107-139] | 97 [84-124] | .34 |
| Atrial fibrillation | 10 (36) | 2 (29) | 8 (67) | .14 |
| Ischemic heart disease | 17 (61) | 5 (71) | 10 (83) | .36 |
| LVEF, % | 26 [21-31] | 27 [23-28] | 30 [23-34] | .46 |
| Baseline treatment | ||||
| β-Blockers | 27 (96) | 7 (100) | 11 (92) | .66 |
| ACEIs | 19 (68) | 4 (57) | 8 (67) | .87 |
| ARBs | 4 (14) | 2 (29) | 3 (25) | .58 |
| MRAs | 27 (96) | 7 (100) | 11 (95) | .66 |
| Nitrates | 6 (21) | 3 (43) | 2 (17) | .40 |
| Hydralazine | 5 (18) | 1 (14) | 3 (25) | .82 |
| Furosemide | 23 (82) | 7 (100) | 12 (100) | .15 |
| CRT | 6 (21) | 2 (29) | 1 (8) | .50 |
| Baseline right catheterization | ||||
| mBP, mmHg | 74 [67-77] | 81 [74-88] | 82 [67-88] | .05 |
| mPAP, mmHg | 39 [35-42] | 44 [38-53] | 51 [47-58] | <.001 |
| PCWP, mmHg | 26 [20-32] | 30 [12-32] | 29 [24-33] | .52 |
| Cardiac index, L/m2 | 1.9 [1.6-2.5] | 1.9 [1.4-2] | 2.1 [1.7-2.8] | .41 |
| sPAP, mmHg | 62 [55-66] | 70 [53-84] | 79 [72-88] | .008 |
| TPG, mmHg | 13 [9-16] | 21 [16-26] | 22 [20-26] | <.001 |
| PVR, WU | 3.37 [2.7-4.6] | 5.9 [5-8.7] | 6.2 [4.9-8.2] | .001 |
| Subsequent treatment | ||||
| Sildenafil | 19 (68) | 7 (100) | 12 (100) | .02 |
| Sildenafil dose, mg/d | 60 [30-120] | 240 [120-240] | 120 [60-160] | .008 |
| LVAD | 5 (18)2 EXCOR1 HVAD2 HeartMate 3 | 7 (100)4 HVAD3 HeartMate 3 | 3 (25)3 EXCOR | <.001 |
| Transplant at 2 y | 21 (75) | 6 (85.7) | 6 (50) | .178 |
| Time to transplant, d | 171 [82-280] | 219.5 [170-313] | 57.5 [42-117] | .118 |
ARBs, angiotensin II receptor blockers; ACEIs, angiotensin conversion enzyme inhibitors; CRT, cardiac resynchronization therapy; GTP, mPAP-PCWP transpulmonary gradient; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; mBP, mean blood pressure; mPAP, mean pulmonary arterial pressure; MRAs, mineralocorticoid receptor antagonists; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance [GTP/cardiac index]; sPAP, systolic pulmonary artery pressure; WU, Wood units.
Values are expressed No. (%) or as median [interquartile range].
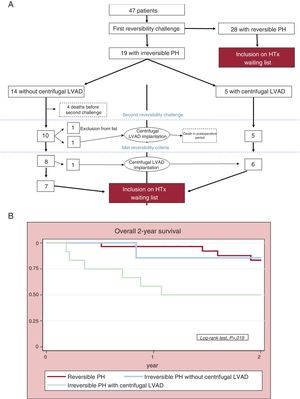
Forty-seven patients were included: 28 with reversible PH and 19 with irreversible PH at baseline right heart catheterization. The breakdown of patients and their outcomes throughout the study period are shown in figure 1A. All 5 patients with irreversible PH treated with centrifugal LVAD implantation after the first vasodilator challenge survived to undergo a second challenge and met the reversibility criteria. Of the 14 patients who did not receive a centrifugal LVAD after the first challenge, just 10 were alive at the time of the second challenge and 8 of them met the reversibility criteria (57% vs 100% in the centrifugal LVAD group, P=.07). These differences can largely be explained by the high proportion of patients who did not undergo centrifugal LVAD implantation and who died before the second challenge.
The Kaplan-Meier 2-year survival curves comparing the 3 groups are shown in figure 1B. Mortality was higher in the group of patients with irreversible PH who did not receive a centrifugal LVAD (6 deaths) than in patients treated with centrifugal LVAD implantation (1 death) and patients with reversible PH after the first challenge (4 deaths) (P=.019). The subanalysis of patients who underwent HTx showed no significant differences (P=.238).
Despite the limitations of this small, single-center, observational study, our results show that HTx candidates with irreversible PH who undergo centrifugal LVAD implantation survive longer than patients not undergoing this procedure and have a similar prognosis to patients with reversible PH. The 2-year mortality rate in patients with irreversible PH not treated with centrifugal LVAD implantation was 50%. The higher survival rate in patients treated with centrifugal LVAD implantation can largely be attributed to their increased likelihood of meeting the reversibility criteria and being added to an HTx waiting list following this procedure. Our results also support previous findings showing that the combined use of sildenafil and centrifugal LVAD implantation is safe.6
Centrifugal LVAD implantation can safely and effectively reverse PH in HTx candidates with an initially negative vasodilator response, providing a bridge to HTx candidacy and a similar prognosis to that of patients with reversible PH.
Conflicts of InterestJ. González-Costello has received consultancy fees from Abbott.