Percutaneous occlusion of the left atrial appendage (LAA) is an alternative to oral anticoagulation therapy for the prevention of ischemic stroke in patients with nonvalvular atrial fibrillation.1
Currently, the 2 devices most frequently used for percutaneous LAA occlusion are the WatchmanTM system (Boston Scientific) and the AmplatzerTM Cardiac Plug (St. Jude Medical). A recently designed second generation of the AmplatzerTM Cardiac Plug, the AmuletTM device (St. Jude Medical), introduces modifications to facilitate device implantation and reduce complications.2
The AmuletTM device obtained the European Economic Area CE mark in January 2013, and in February 2013 the device received a restricted launch in selected centers, producing good results.2,3 However, difficulties were encountered with the release of a new internal delivery cable, forcing the company to halt distribution in July of the same year. After modification of the cable, the AmuletTM device was relaunched in a restricted setting in October 2014, and to our knowledge, there has been no published report to date on its safety and short-term efficacy.
The study included all consecutive patients undergoing percutaneous LAA with the AmuletTM device at 2 centers between October and December 2014. Procedures were performed as described,3 and clinical and echocardiographic follow-up was scheduled for 2 to 3 months after the procedure.
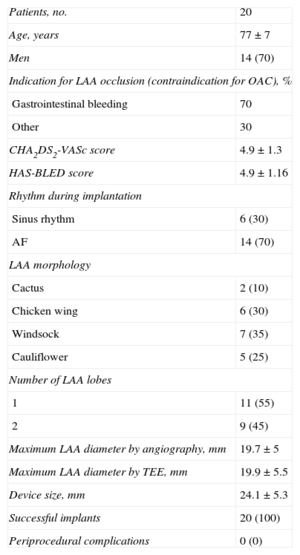
A total of 20 patients were included; population and procedure characteristics are shown in the Table.
Study Population and Procedure Characteristics
| Patients, no. | 20 |
| Age, years | 77±7 |
| Men | 14 (70) |
| Indication for LAA occlusion (contraindication for OAC), % | |
| Gastrointestinal bleeding | 70 |
| Other | 30 |
| CHA2DS2-VASc score | 4.9±1.3 |
| HAS-BLED score | 4.9±1.16 |
| Rhythm during implantation | |
| Sinus rhythm | 6 (30) |
| AF | 14 (70) |
| LAA morphology | |
| Cactus | 2 (10) |
| Chicken wing | 6 (30) |
| Windsock | 7 (35) |
| Cauliflower | 5 (25) |
| Number of LAA lobes | |
| 1 | 11 (55) |
| 2 | 9 (45) |
| Maximum LAA diameter by angiography, mm | 19.7±5 |
| Maximum LAA diameter by TEE, mm | 19.9±5.5 |
| Device size, mm | 24.1±5.3 |
| Successful implants | 20 (100) |
| Periprocedural complications | 0 (0) |
AF, atrial fibrillation; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly [> 65 years], drugs/alcohol concomitantly; LAA, left atrial appendage; OAC, oral anticoagulation therapy; TEE, transesophageal echocardiogram.
Values are expressed as no. (%) or mean±standard deviation.
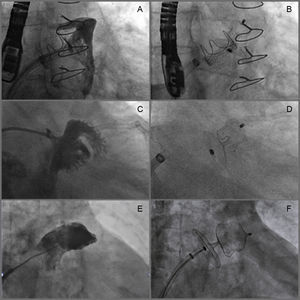
Implantation was successful in 100% of the patients, despite the complicated morphology of some appendages (Figure). There were no periprocedural cases of device embolization, pericardial effusion, stroke, acute myocardial infarction, or death.
Examples of LAA occlusion with the Amulet device. A and B: Small LAA (maximum diameter 12mm) with “chicken wing” morphology; a 16-mm Amulet device was implanted, producing good results. C and D: Early bifurcating LAA with a long and very narrow lobe (maximum diameter 11mm) and no well-defined landing zone; a 16-mm Amulet device was implanted, producing good results. E and F: Large LAA (landing zone 30 mm) with “windsock” morphology; a 34 mm Amulet device was implanted, producing good results. LAA, left atrial appendage.
All patients underwent clinical follow-up examination after a median postprocedure interval of 60 days (interquartile range, 48-80 days). One patient had a transient ischemic attack without sequels, likely related to the patient's decision to prematurely terminate antiplatelet therapy, and another patient was diagnosed a few weeks after the procedure with pericarditis, which resolved with corticoid therapy.
Echocardiographic follow-up was completed in 100% of the patients. Complete sealing of the LAA was observed in all patients, with no peridevice leaks, thrombosis, or pericardial effusion.
The present study thus demonstrates that percutaneous LAA occlusion with the new AmuletTM device is feasible, safe, and shows efficacy in short-term follow-up.
The AmuletTM device is distinguished from the AmplatzerTM Cardiac Plug by several key modifications: the new device is fully preloaded within the delivery system, the disc diameters are larger, the connecting waist between the lobe and disc is longer, the lobe is longer and is available in wider diameters (up to 34mm), there are more stabilizing wires, the device features a new delivery cable with a distal cone, and the distal and proximal end-screws do not protrude after release.2
In our study, the AmuletTM device successfully occluded the LAA in all patients, despite the challenging anatomies encountered (10% of appendages had a maximum diameter ≥ 30 mm and 30% had a “chicken-wing” morphology). Because of the larger lobe, increased number of stabilizing wires, and longer waist between lobe and disc, the lobe can be positioned deeper in the LAA while keeping the proximal disc outside of the LAA ostium, thus favoring complete closure even of complex LAA structures such as the “chicken-wing” morphology.3
The success achieved here with the AmuletTM device is greater than that reported for the WatchmanTM system and the AmplatzerTM Cardiac Plug,1,4 and is similar to that reported by other investigators.2,3 We observed no periprocedural complications. These findings can be explained by our accumulated expertise in percutaneous LAA occlusion and the new modifications introduced in the AmuletTM device. Follow-up transesophageal echocardiography detected no complications (device embolization, thrombosis, or pericardial effusion).
The major limitations of this study are its observational design and the small number of patients. When interpreting the excellent results, it is important to consider that the procedures were performed in centers with experience in LAA occlusion. Given the small number of patients and the short-term follow-up, it is not possible to establish a direct link between the nondetection of thrombi or complications by transesophageal echocardiography and the design of the new device. Nonetheless, to our knowledge, this is the first study published on LAA occlusion with the AmuletTM device since the modification of the cable and is one of the most extensive studies with the device so far.
CONFLICTS OF INTERESTIgnacio Cruz-González is proctor and consultant for St. Jude Medical and Boston Scientific. Dabit Arzamendi is proctor and consultant for St. Jude Medical.