Major research on insertable cardiac monitors (ICMs) has focused on simplifying the insertion procedure while increasing ICM performance with more accurate detection algorithms. The marked size reduction of ICMs has allowed minimally invasive insertion in the subcutaneous tissue.1 These improvements have opened the door for this procedure to be performed by qualified professionals such as certified nurses, which could result in more efficient time and resource management, potentially reducing waiting lists.1
“ICM nurse” is an ongoing multicenter, prospective, single-arm, open-label study to assess the safety and efficacy of the ICM BIOMONITOR III and IIIm (Biotronik, Germany) at 2 centers in Spain. This interim analysis presents short-term data on the feasibility of ambulatory nurse-led ICM insertions by showing acute procedural success, wound healing, and ICM performance. Data were collected at baseline, at patient discharge, and after 1 week (on-site or remotely as per routine clinical practice). Insertion results were compared with retrospective data on insertions performed by physicians. Patients rated their satisfaction with the procedure performed by nurses via a 12-item purpose-made questionnaire. The study was approved by the ethics committee of Hospital Puerta de Hierro (Madrid, Spain). All participants provided informed consent.
Inclusion criteria consisted of age> 18 years and an indication for ICM insertion. Exclusion criteria were an existing axillary or inframammary implant, indication for pacemaker or implantable cardioverter-defibrillator, compromised immunological system or risk of developing an infection, participation in another interventional study, pregnancy or breastfeeding, life expectancy <12 months, or thoracic anatomy that could compromise the procedure.
BIOMONITOR III and IIIm have a longer sensing vector and a miniaturized profile (77.5mm x 8.6mm x 4.6mm; 4g) that enables 1-step insertion. Insertions were performed using local anesthesia, following the routine procedures of participating centers.
Forty-seven participants were included in the study (ICM implantations were performed by nurses in 20 patients and by physicians in 27), and insertion was successful in all (table 1). Insertions in the nurse group were performed by 3 nurses (2 at Hospital General Universitario Dr Balmis de Alicante and 1 at Hospital Universitario Puerta de Hierro de Madrid) with a mean experience of 9.7 years in the electrophysiology laboratory.
Clinical and demographic characteristics and insertion data of the study participants
| Variables | Nursen=20 | Physiciann=27 | P |
|---|---|---|---|
| Age, y | 63.4±10.8 | 74.2±11.8 | .002 |
| Weight, kg, | 79.4±20.6 | 72.5±11.2 | .187 |
| NYHA classa(n=46) | .0001 | ||
| I | 17 (89.5) | 7 (25.9) | |
| II | 1 (5.3) | 15 (55.6) | |
| II-III | 1 (5.3) | 3 (11.1) | |
| III | 0 | 2 (7.4) | |
| ICM indicationb | .0001 | ||
| Suspected AF | 1 (5.0) | 2 (7.4) | |
| Syncope of unknown cause | 7 (35.0) | 6 (22.2) | |
| Recurrent palpitations | 1 (5.0) | 3 (11.1) | |
| Cryptogenic stroke | 11 (55.0) | 0 | |
| Post-AF ablation monitoring | 1 (5.0) | 1 (5.0) | |
| Suspicion of cardiac conduction disorder | 0 | 5 (18.5) | |
| Other | 0 | 11 (40.7) | |
| Symptoms | 7 (35.0) | 20 (74.1) | .007 |
| Syncope | 5 (71.4) | 6 (30.0) | |
| Dizziness | 1 (14.3) | 9 (45.0) | |
| Palpitations | 1 (14.3) | 3 (15.0) | |
| Dyspnea | 0 | 2 (10.0) | |
| History of AF | |||
| None | 17 (85.0) | 18 (66.7) | .121 |
| Paroxysmal | 3 (15.0) | 4 (14.8) | |
| Persistent | 0 | 5 (18.5) | |
| Previous AF ablation | 1 (5.0) | 0 | .240 |
| History of thromboembolic events or stroke | .0001 | ||
| None | 10 (50.0) | 25 (92.6) | |
| Stroke | 9 (45.0) | 2 (7.4) | |
| Transient ischemic attack | 1 (5.0) | 0 | |
| Comorbiditiesb | |||
| COPD | 4 (20.0) | 1 (3.7) | .073 |
| Diabetes mellitus | 3 (15.0) | 6 (22.2) | .638 |
| Stroke | 11 (55.0) | 2 (7.4) | .0001 |
| Hypertension | 8 (40.0) | 20 (74.1) | .019 |
| Hypercholesterolemia | 9 (45.0) | 13 (48.1) | .831 |
| Obesity | 6 (30.0) | 4 (14.8) | .209 |
| Renal insufficiency | 2 (10.0) | 6 (22.2) | .270 |
| Liver insufficiency | 2 (10.0) | 1 (3.7) | .383 |
| Family history of cardiovascular disease | 6 (30.0) | 7 (41.2) | .478 |
| Insertion position | |||
| Position A | 7 (35.0) | 19 (70.4) | .016 |
| Position B | 13 (65.0) | 8 (29.6) | |
| Caudal-cranial | 7 (35.0) | 20 (74.1) | .007 |
| Cranial-caudal | 13 (65.0) | 7 (25.9) | |
| Insertion time (skin cut to wound closure) | .010 | ||
| <1 min | 1 (5.0) | 0 | |
| 1-5 min | 12 (60.0) | 6 (22.2) | |
| > 5 min | 7 (35.0) | 21 (77.8) | |
| Insertion time (anesthesia to insertion tool removal) | .339 | ||
| <5 min | 8 (40.0) | 8 (29.6) | |
| 5-10 min | 9 (45.0) | 18 (66.7) | |
| 10-15 min | 2 (10.0) | 1 (3.7) | |
| 15-20 min | 1 (5.0) | 0 | |
| Ease of insertion | .008 | ||
| Very easy | 5 (25.0) | 17 (63.0) | |
| Easy | 9 (45.0) | 10 (37.0) | |
| Normal | 5 (25.0) | ||
| Difficult | 1 (5.0) | ||
| Wound closure methods | .027 | ||
| Adhesive strips | 11 (55.0) | 24 (88.9) | |
| Staples | 8 (40.0) | 3 (11.1) | |
| Intradermal stitches | 1 (5.0) | 0 | |
| Complications | |||
| None | 17 (85.0) | 27 (100.0) | |
| Insertion site pain | 3 (15.0) | ||
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; ICM; insertable cardiac monitor; NYHA, New York Heart Association.
The data are expressed as No. (%) or mean±standard deviation.
The time from skin cut to wound closure was> 5minutes in 35.0% of the insertions performed by nurses and in 77.8% of those performed by physicians (P=.010). The wound was closed by adhesive strips in 55.0% of the insertions performed by nurses vs 88.9% of those carried out by physicians, by staples in 40.0% vs 11.1% of insertions, and by intradermal stitches in 5.0% vs 0% of insertions (P=.027). The insertion procedure was rated as ‘very easy/easy’ by nurses in 70.0% of cases and in 100% of cases by physicians (table 1).
The only complication reported during the procedure was insertion site pain (3 patients in the nurse group, 15.0%) (table 1). Only 1 wound-related adverse event was reported (device migration) in the nurse group after 1 week of device insertion, followed by an external device repositioning. The ICM was explanted in 1 patient due to detection of atrial fibrillation.
The mean R-wave amplitude was 0.60±0.24mV in the nurse group and 0.56±0.31 in the physician group at device insertion (P=.274). P-waves were visible in 19 (95.0%) patients in insertions performed by nurses and in 20 (74.1%) patients in insertions performed by physicians at device insertion (P=.059).
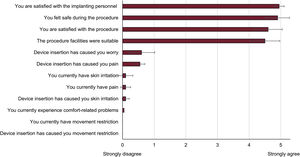
Overall patient satisfaction with insertions performed by nurses was high: implanting personnel (4.95±0.22), facilities (4.50±0.89) and safety (4.90±0.31) (figure 1).
The device detected 3 arrhythmias in the nurse group: 2 episodes of atrial fibrillation and 1 high ventricular rate. One patient showed paroxysmal atrial fibrillation with recurrent episodes that resulted in a supraventricular episode with rapid ventricular response, and another patient showed atrial fibrillation detected 1 week after device insertion.
This is the first clinical study suggesting that certified nurses can safely insert the BIOMONITOR III and IIIm in an ambulatory setting by using a short and simple procedure, with high patient and implanter satisfaction, no severe acute complications, and large signal amplitudes. The main limitations of the study are the small patient cohort analyzed and the comparison of data with a retrospective cohort.
The time from skin cut to wound closure (1-5min in 60% of insertions performed by nurses vs 22.2% performed by physicians) is within the range of 3.6minutes previously reported with the BIOMONITOR III implanted by physicians in cardiac catheterization laboratories2 and is substantially lower than the 7 to 9minutes registered with the BioMonitor 2 inserted in cardiac catheterization laboratories.3 Differences in procedure length between nurses and physicians could be explained considering that physicians mostly performed the procedure in the operating room and nurses in an ambulatory setting.
Patients showed a high level of satisfaction with the procedure, with satisfaction being highest with the implanting personnel, followed by the safety felt during the procedure. The finding that the most valued aspect of the procedure was the implanting personnel could be due to the critical role played by nurses in establishing closer contact with patients. Moreover, the ambulatory setting could also be a more relaxed and less intimidating environment for the patient.
Safety data suggest no added risk for insertions performed by certified nurses, as only 3 patients experienced insertion site pain and there was only 1 wound-related adverse event, supporting the low complication rates previously reported in insertions performed by nurses and advanced practice providers.4
The mean R-wave amplitude of 0.60mV supports the acute procedural success of insertions performed by nurses. P-waves were visible in 95.0% patients at device insertion, contrasting with the 58% reported with the Reveal LINQ (Medtronic Inc, United States)5 and 67% to 72% with the BioMonitor 2.6
In conclusion, the miniaturized implantable cardiac monitor BIOMONITOR III can be efficiently and safely inserted by certified nurses.
FUNDINGThis work was supported by the Research Agency of the Spanish Society of Cardiology. The Research Agency of the Spanish Society of Cardiology received an unrestricted research grant from BIOTRONIK SE & Co. KG (Berlin, Germany). Medical Writing services were supported by BIOTRONIK SE & Co. KG (Berlin, Germany).
AUTHORS’ CONTRIBUTIONSConcept and design: J.G. Martínez, I. Fernández Lozano. Acquisition, analysis, or interpretation of data: J.G. Martínez, J. de Andrés, I. Lillo, D. Veloza, H. Reig, I. Fernández Lozano. Drafting of the manuscript: J.G. Martínez, I. Fernández Lozano. Revision of the manuscript: J.G. Martínez, J. de Andrés, I. Lillo, D. Veloza, H. Reig, I. Fernández Lozano.
CONFLICTS OF INTERESTJ.G. Martínez has received honoraria for participating in advisory boards and presentations from Abbott. I. Fernández Lozano has received fees for fellowships from Fundación de Investigación Hospitalaria and Sociedad Española de Cardiología (SEC), and for clinical studies from Biotronik and Abbott. The remaining authors have no conflicts of interest to disclose.
The authors thank Alicia Moreno and Berta Mateos for the technical and statistical support provided. Carla Granados from Trialance SCCL provided medical writing assistance.