Percutaneous aortic valve implantation is currently an alternative to surgical valve replacement in patients with severe symptomatic aortic stenosis at high surgical risk or with contraindications for surgery.1 The ACURATE-Neo TF self-expandable aortic valve (Symetis SA, Lausanne, Switzerland) was approved for implantation in Europe in late 2014. We analyzed the initial experience related to all ACURATE-Neo TF prostheses implanted in the Iberian Peninsula to date.
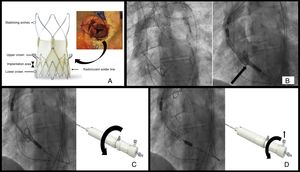
ACURATE-Neo TF is a porcine pericardial tissue valve mounted on a nitinol stent consisting of several parts (Figure A): 3 stabilizing arches that allow the valve to self-align during implantation to ensure final coaxiality of the valve with the native annulus; the upper crown–that is, the central segment of the scaffold where the leaflets are sutured–which functions as a supra-annular valve (the inverted hook configuration directs the native valve leaflets toward the annulus, decreasing the possibility of occluding the coronary ostia and reducing the degree of paravalvular leak); and the lower crown, the most distal segment, which is implanted with minimal extension into the left ventricular outflow tract to decrease the incidence of conduction disturbances. The inner and outer surfaces of this part of the prosthesis are also covered with pericardial tissue to seal against paravalvular leak. The soldering material located in this segment generates a radiolucent line while the valve is encased within the release system and serves as a guide during implantation. ACURATE-Neo TF comes in 3 sizes–small, medium, and large–which are suitable to treat aortic annuli diameters ranging from 21 to 27mm.
A: structure of the prosthetic valve; the arrow indicates the position of the upper crown and its relationship with the native valve leaflets in a real case. B: initial position. C: release, first step; the curved arrow indicates the direction of rotation of the distal knob; D: release, second step and deployment; the vertical arrow indicates the safety button that must be removed and the curved arrow the direction of rotation of the proximal knob.
The prosthesis is compressed within 2 sheaths at the distal end of the delivery catheter, and release is carried out in 2 steps. To advance the catheter, an 18-Fr introducer is required or a 13- to 15-Fr expandable introducer for the 3 sizes. The release handle contains 2 rotational knobs: the distal knob releases the stabilizing arches and upper crown, whereas the proximal knob releases the lower crown, at which time the valve is completely deployed. To prevent inadvertent release of the lower crown, the handle contains a safety button that must be removed to allow rotation of the proximal knob.
Aortic balloon valvuloplasty is recommended prior to implantation. Once the valve is advanced to the native annulus, 2-step deployment occurs from the top down; that is, from the aorta to the left ventricle, unlike other self-expanding valves. It is important to know that the valve is not retrievable.
Initial position: As in other percutaneous prostheses, the 3 coronary sinuses must be aligned to identify the valvular annulus. This is used as a reference to guide positioning of the implant, which should extend between 4 and 6mm into the left ventricular outflow tract. To achieve this objective, the radiopaque solder marker should be aligned with the annulus (Figure B).
Step 1: Once the initial position is reached, the upper crown and stabilizing arches are consecutively released by rotating the distal knob on the handle in a counterclockwise direction. At this point, the valve position can still be readjusted by gently retracting or pushing the catheter forward. During these maneuvers, it is important not to insert the prosthesis into the left ventricle with the upper crown deployed; given its configuration, it will be very difficult to return it to the aorta. (Figure C).
Step 2: Finally, the lower crown is released and the prosthesis is fully deployed and functioning. Although the prosthesis is already very stable in this last step, overpacing can be useful to avoid undesirable final movements. The lower crown is released by rotating the proximal knob in a counterclockwise direction after removing the safety button (Figure D).
Once the prosthesis has been deployed, the catheter is retrieved from the left ventricle and the device is re-encapsulated in the descending aorta by rotating the 2 knobs in the opposite order and direction to that used for releasing the prosthesis.
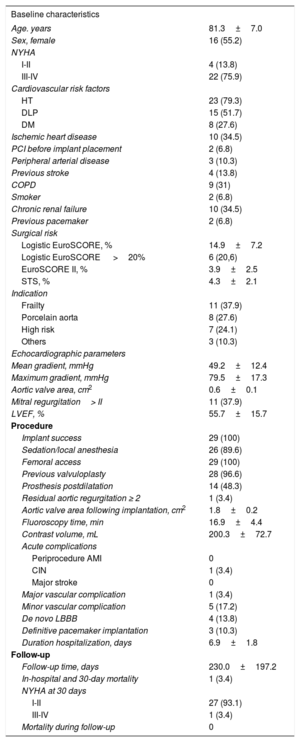
We present the outcome of ACURATE-Neo TF use in 29 patients, 28 with severe aortic stenosis and 1 with severe aortic failure due to dysfunction of a surgical prosthesis. The baseline characteristics of the patients, procedure-related data, and follow-up information are provided in Table.
Baseline and Procedure-related Variables
| Baseline characteristics | |
|---|---|
| Age. years | 81.3±7.0 |
| Sex, female | 16 (55.2) |
| NYHA | |
| I-II | 4 (13.8) |
| III-IV | 22 (75.9) |
| Cardiovascular risk factors | |
| HT | 23 (79.3) |
| DLP | 15 (51.7) |
| DM | 8 (27.6) |
| Ischemic heart disease | 10 (34.5) |
| PCI before implant placement | 2 (6.8) |
| Peripheral arterial disease | 3 (10.3) |
| Previous stroke | 4 (13.8) |
| COPD | 9 (31) |
| Smoker | 2 (6.8) |
| Chronic renal failure | 10 (34.5) |
| Previous pacemaker | 2 (6.8) |
| Surgical risk | |
| Logistic EuroSCORE, % | 14.9±7.2 |
| Logistic EuroSCORE >20% | 6 (20,6) |
| EuroSCORE II, % | 3.9±2.5 |
| STS, % | 4.3±2.1 |
| Indication | |
| Frailty | 11 (37.9) |
| Porcelain aorta | 8 (27.6) |
| High risk | 7 (24.1) |
| Others | 3 (10.3) |
| Echocardiographic parameters | |
| Mean gradient, mmHg | 49.2±12.4 |
| Maximum gradient, mmHg | 79.5±17.3 |
| Aortic valve area, cm2 | 0.6±0.1 |
| Mitral regurgitation> II | 11 (37.9) |
| LVEF, % | 55.7±15.7 |
| Procedure | |
| Implant success | 29 (100) |
| Sedation/local anesthesia | 26 (89.6) |
| Femoral access | 29 (100) |
| Previous valvuloplasty | 28 (96.6) |
| Prosthesis postdilatation | 14 (48.3) |
| Residual aortic regurgitation ≥ 2 | 1 (3.4) |
| Aortic valve area following implantation, cm2 | 1.8±0.2 |
| Fluoroscopy time, min | 16.9±4.4 |
| Contrast volume, mL | 200.3±72.7 |
| Acute complications | |
| Periprocedure AMI | 0 |
| CIN | 1 (3.4) |
| Major stroke | 0 |
| Major vascular complication | 1 (3.4) |
| Minor vascular complication | 5 (17.2) |
| De novo LBBB | 4 (13.8) |
| Definitive pacemaker implantation | 3 (10.3) |
| Duration hospitalization, days | 6.9±1.8 |
| Follow-up | |
| Follow-up time, days | 230.0±197.2 |
| In-hospital and 30-day mortality | 1 (3.4) |
| NYHA at 30 days | |
| I-II | 27 (93.1) |
| III-IV | 1 (3.4) |
| Mortality during follow-up | 0 |
AMI, acute myocardial infarction; CIN, contrast-induced nephropathy; COPD, chronic obstructive pulmonary disease; DLP, dyslipidemia; DM, diabetes mellitus; EuroSCORE, European System for Cardiac Operative Risk Stratification; HT, hypertension; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons.
Continuous variables are expressed as the mean±deviation and categorical values as No. (%).
In our initial series of 29 patients and in line with the currently available evidence compiled in registries and in small real-world reports,2,3 percutaneous implantation of the ACURATE-neo TF aortic valve prosthesis was safe and was associated with a low incidence of complications and mortality at 30 days. The results were particularly good in terms of residual paravalvular leak and requirement for pacemaker implantation in comparison with other self-expandable valves. The larger profile and smaller range of sizes relative to other valves make ACURATE-neo TF accessible to a smaller number of patients, and the incidence of vascular complications (mainly minor ones) was somewhat higher.
The design of the upper crown, explained above, makes this aortic valve prosthesis particularly indicated for patients with low positioning of the coronary ostia or with aortas with narrow sinuses of Valsalva, as the risk of coronary occlusion is higher in these cases.
.