Some patients cannot receive aspirin, and are therefore unable to receive dual antiplatelet therapy (DAPT) following a percutaneous intervention. For these patients, antiplatelet monotherapy (APM) with a P2Y12 inhibitor (iP2Y12) is a potential option, although there is little published data on this therapy. Our aim was to investigate the incidence of APM use in clinical practice and to determine the outcome in this group compared with that in patients receiving DAPT. From August 2008 to April 2016, we enrolled all patients receiving APM (ticlopidine 150mg/12h, clopidogrel 75mg/24h, ticagrelor 90mg/12h, or prasugrel 10mg/24h) following angioplasty. Patients receiving anticoagulant agents at hospital discharge or during follow-up (according to telephone contact or medical visit) were excluded, leaving a total of 37 patients for the study.
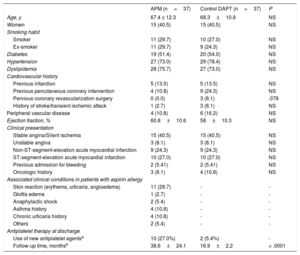
Clinical variables and the reasons for APM use were recorded (Table). The incidence of APM use was 0.42% with a median follow-up of 48.8 months; 27% had received one of the newer antiplatelet agents (6, prasugrel; 4, ticagrelor). Patients receiving APM were matched (1:1) with a control group given DAPT according to the standard clinical practice, selected from 1438 consecutive patients undergoing stent placement in our center between 2011 and 2013. The criteria for matching were age, sex, hypertension, dyslipidemia, diabetes, smoking, clinical presentation, type of stent (drug-eluting/metallic), and ejection fraction. Among the controls, 2 received prasugrel. Compared with patients given DAPT, the group receiving APM showed no significant differences in the rate of major cardiovascular events at 3 years (mortality, reinfarction, or revascularization requirement; P=.810) (Figure). In both groups, major cardiovascular events occurred mainly within the first year. Four patients in each group died during follow-up. There was 1 major bleeding event in the DAPT group and none in the APM group during follow-up (nonsignificant difference).
Baseline Characteristics of the Patients Included, and Associated Clinical Conditions in Patients With Aspirin Allergy
| APM (n=37) | Control DAPT (n=37) | P | |
|---|---|---|---|
| Age, y | 67.4 ± 12.3 | 68.3±10.8 | NS |
| Women | 15 (40.5) | 15 (40.5) | NS |
| Smoking habit | |||
| Smoker | 11 (29.7) | 10 (27.0) | NS |
| Ex-smoker | 11 (29.7) | 9 (24.3) | NS |
| Diabetes | 19 (51.4) | 20 (54.0) | NS |
| Hypertension | 27 (73.0) | 29 (78.4) | NS |
| Dyslipidemia | 28 (75.7) | 27 (73.0) | NS |
| Cardiovascular history | |||
| Previous infarction | 5 (13.5) | 5 (13.5) | NS |
| Previous percutaneous coronary intervention | 4 (10.8) | 9 (24.3) | NS |
| Pervious coronary revascularization surgery | 0 (0.0) | 3 (8.1) | .078 |
| History of stroke/transient ischemic attack | 1 (2.7) | 3 (8.1) | NS |
| Peripheral vascular disease | 4 (10.8) | 6 (16.2) | NS |
| Ejection fraction, % | 60.8±10.6 | 58±10.3 | NS |
| Clinical presentation | |||
| Stable angina/Silent ischemia | 15 (40.5) | 15 (40.5) | NS |
| Unstable angina | 3 (8.1) | 3 (8.1) | NS |
| Non-ST-segment-elevation acute myocardial infarction | 9 (24.3) | 9 (24.3) | NS |
| ST-segment-elevation acute myocardial infarction | 10 (27.0) | 10 (27.0) | NS |
| Previous admission for bleeding | 2 (5.41) | 2 (5.41) | NS |
| Oncologic history | 3 (8.1) | 4 (10.8) | NS |
| Associated clinical conditions in patients with aspirin allergy | |||
| Skin reaction (erythema, urticaria, angioedema) | 11 (29.7) | - | - |
| Glottis edema | 1 (2.7) | - | - |
| Anaphylactic shock | 2 (5.4) | - | - |
| Asthma history | 4 (10.8) | - | - |
| Chronic urticaria history | 4 (10.8) | - | - |
| Others | 2 (5.4) | - | - |
| Antiplatelet therapy at discharge | |||
| Use of new antiplatelet agentsa | 10 (27.0%) | 2 (5.4%) | - |
| Follow-up time, monthsb | 38.6±24.1 | 16.9±2.2 | < .0001 |
APM, antiplatelet monotherapy; DAPT, dual antiplatelet therapy; NS, nonsignificant difference
Understandably, follow-up times were shorter with DAPT than with APM. This is because, after a variable time period, patients receiving DAPT were changed to APM; that is, in the control arm, the mean follow-up of 16.9 months is the time patients were receiving DAPT. Thereafter, clinical follow-up continued with APM, according to the standard clinical practice.
A: Kaplan-Meier survival curve for the combined event, MACE, at 3 years of follow-up. B: Kaplan-Meier survival curve of event-free time (MACE) at 2 years of follow-up in patients with APM treated with clopidogrel and the new iP2Y12 agents.
APM, antiplatelet monotherapy; DAPT, dual antiplatelet therapy; iP2Y12: P2Y12 inhibitors; MACE, major adverse cardiovascular events.
Park et al.1 investigated the development of bleeding with APM–either aspirin or clopidogrel–following DAPT use, and reported that major bleeding rates were similar in the comparison of these 2 agents. Gastrointestinal bleeding is more common with aspirin than clopidogrel, and DAPT with these agents increases the risk of gastrointestinal bleeding by 2- or 3-fold compared with aspirin alone.2 There were no cases of stent thrombosis during follow-up in our study, likely because of the low incidence of this phenomenon (< 1% per year) and the small size of the sample. Although previous studies have indicated that stent thrombosis rates after DAPT discontinuation are significant and are related to discontinuing the second antiplatelet agent, the absence of this event in our study raises the hypothesis that iP2Y12 agents may be able to maintain an adequate antithrombotic state. Data on iP2Y12 monotherapy are scarce and some information is from patients who have undergone early DAPT discontinuation. Ferreira-González et al.3 have indicated that there is a variable relationship in time between occurrence of events and the day of discontinuation. The PARIS registry has shown that APM (understood as early discontinuation of DAPT, which may be temporary or permanent) carried out under medical supervision can provide acceptable results.4
Another finding of our study is related to the type of iP2Y12. We observed a trend toward more favorable outcomes in patients receiving the newer iP2Y12 agents (Figure B), possibly because of greater individual variability in pharmacodynamics, CYP2C19 polymorphisms in patients requiring antiplatelet therapy with clopidogrel, and a more potent antiplatelet action of the newer agents.5
Effective strategies are available in clinical practice for treating patients with aspirin allergy and contraindications for implanting a drug-eluting stent, such as desensitization protocols, likely the preferable option, or substituting aspirin for an analog or another antiplatelet agent (indobufen, trapidil, triflusal).6 Nonetheless, these measures sometimes fail or are not applied for various reasons. Additionally, in populations with an elevated bleeding risk, attempts are made to reduce DAPT to a minimum. A strategy of iP2Y12 monotherapy could be useful in both these scenarios. Results are pending for the TWILIGHT and GLOBAL LEADERS studies, which may establish broader indications for the use of APM.
Our study has the limitations derived from its design and the small number of patients included, as it evaluates an uncommon treatment. Nonetheless, it may be an indication of typical clinical practice.
In conclusion, this study is the first to provide data on patients initially treated with iP2Y12 monotherapy. This therapeutic strategy is not commonly used in clinical practice but is a reasonable choice for patients who cannot receive DAPT containing aspirin, as the outcome at 36 months was similar to that of patients receiving DAPT. Furthermore, the new iP2Y12 agents could be an option when APM is needed, particularly after the acute phase has passed.
CONFLICTS OF INTERESTI.J. Núñez-Gil has participated in lectures for AstraZeneca and Lilly, and has served as an advisor for AstraZeneca. E. Cerrato is a speaker for AstraZeneca Italy and has received research grants from AstraZeneca Spain.
.