Patent ductus arteriosus (PDA) affects 70% of preterm infants and is often associated with severe complications. Postnatal ductal closure is normally regulated by increased oxygen pressure and decreased prostaglandin E2 level, which remains elevated in preterm infants. Cyclooxygenase is a key enzyme in prostaglandin synthesis and therefore cyclooxygenase inhibitors are used to treat PDA.1 However, many patients with PDA do not respond to treatment. Some studies have associated treatment failure with chorioamnionitis and sepsis, which are risk factors related to a systemic inflammatory response.2,3 Inflammation may increase cyclooxygenase-1 activity and prostaglandin E2 production,4 implying that it is an important factor in persistent PDA. The ultrasensitive C-reactive protein test (us-CRP) can be used to accurately quantitate low concentrations of C-reactive protein (CRP), which are currently regarded as accurate markers of low-grade inflammation.5 Therefore, we hypothesized that at the time of diagnosis hs-CRP levels would be higher in patients with PDA than in those without PDA.
We conducted a retrospective study of all preterm infants who weighed ≤ 1500 grams, had a gestational age of ≤ 30 weeks, and were born between January 1, 2009 and December 31, 2014 in the Hospital Privado Universitario de Córdoba (Argentina). Exclusion criteria consisted of congenital, chromosomal, or genetic abnormalities, premature death, twins, sepsis, necrotizing enterocolitis, and intraventricular and intestinal hemorrhage. During the study period, all preterm infants underwent echocardiography on the third day of life. Significant PDA was defined as a ductal diameter > 1.5 mm and the presence of at least 2 of the following criteria: left atrium/aorta ratio > 1.5, pulsatile flow in the arterial duct, retrograde or absent diastolic flow in the anterior cerebral artery or descending aorta, or fractional shortening < 40%. Data were collected on weight, gestational age, gender, chorioamnionitis, respiratory distress syndrome, and the use of prenatal corticosteroids. High-sensitivity C-reactive protein was measured with a detection limit of 0.06 mg/dL.
Variables are expressed as 95% confidence intervals (95%CI), means (standard deviation), or medians (95%CI) according to the formula M=n−tαn+1/2 and M=n+tαn−1/2. Differences were determined using the Student t test, Mann-Whitney U test, and Fisher exact test as needed. A P value of < .05 was used as a cutoff for statistical significance.
The study was approved by the Institutional Ethics Committee and the confidentiality of patient data was maintained.
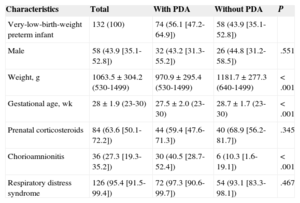
Of 138 eligible patients, 4 were excluded due to death and 2 due to incomplete data; thus, the final sample comprised 132 patients. Eight of the 74 patients with PDA died (10.8%; 95%CI, 3.1 to 18.6) and 4 of the 58 patients without PDA died (6.9%; 95%CI, 1.9 to 16.7) (P = .637). The Table shows the baseline clinical characteristics of the 2 groups and their differences. Patients with PDA had significantly lower weight and gestational age, and a significantly higher rate of chorioamnionitis.
Bivariate Analysis of the Clinical Characteristics of the Study Patients
| Characteristics | Total | With PDA | Without PDA | P |
|---|---|---|---|---|
| Very-low-birth-weight preterm infant | 132 (100) | 74 (56.1 [47.2-64.9]) | 58 (43.9 [35.1-52.8]) | |
| Male | 58 (43.9 [35.1-52.8]) | 32 (43.2 [31.3-55.2]) | 26 (44.8 [31.2-58.5]) | .551 |
| Weight, g | 1063.5±304.2 (530-1499) | 970.9±295.4 (530-1499) | 1181.7±277.3 (640-1499) | < .001 |
| Gestational age, wk | 28±1.9 (23-30) | 27.5±2.0 (23-30) | 28.7±1.7 (23-30) | < .001 |
| Prenatal corticosteroids | 84 (63.6 [50.1-72.2]) | 44 (59.4 [47.6-71.3]) | 40 (68.9 [56.2-81.7]) | .345 |
| Chorioamnionitis | 36 (27.3 [19.3-35.2]) | 30 (40.5 [28.7-52.4]) | 6 (10.3 [1.6-19.1]) | < .001 |
| Respiratory distress syndrome | 126 (95.4 [91.5-99.4]) | 72 (97.3 [90.6-99.7]) | 54 (93.1 [83.3-98.1]) | .467 |
PDA, patent ductus arteriosus.
Data are expressed as no. (%), 95% confidence interval, or mean±standard deviation [interquartile range].
On the third day, the median hs-CRP level was 0.47 mg/dL (95%CI, 0.36 to 0.58) in patients with PDA vs 0.18 mg/dL (95%CI, 0.05 to 0.31) in those without PDA (P < .001). On the seventh day, the median hs-CRP level was 0.42 mg/dL (95%CI, 0.31 to 0.53) in patients with PDA vs 0.35 mg/dL (95%CI, 0.22 to 0.48) and in those without PDA (P = .230).
To our knowledge, this is the first study to investigate the relationship between hs-CRP and PDA. We found that PDA patients have significantly higher levels of hs-CRP. This finding would support the hypothesis that patients with PDA exhibit an inflammatory response,5 which increases cyclooxygenase-1 activity and prostaglandin E2 production, and maintains PDA.4 Patients with sepsis were not included, which excludes bacterial infection as a cause of inflammation. The intense oxidative stress observed in patients with PDA6 could be caused by inflammation or by any remaining prenatal inflammation. In this study, chorioamnionitis was a significant factor in the persistence of PDA. This finding has been reported elswhere.4
This study is limited by its retrospective nature and the relatively small sample size. We attempted to minimize selection and information bias by the use of strict definitions and statistical cutoffs. We calculated that 44 patients per group would be needed for a sample size with sufficient power to detect a difference of 0.30 mg/dL of hs-CRP. Although the sample had sufficient power, the results should be confirmed by larger prospective studies.