Cardiac magnetic resonance imaging (MRI) is an established diagnostic tool in medicine. To improve image quality, gadolinium-based chemicals are commonly used. Since gadolinium-based contrast agents (GBCA) are primarily excreted by the kidney, patients with decreased kidney function showed an increased risk of GBCA retention leading to a potential risk of nephrogenic systemic fibrosis. In the last few years, magnetic resonance signal hyperintensity due to gadolinium retention was observed in the central nervous system in patients having received multiple GBCA doses over time even in patients with normal renal function.1 This resulted in warnings by the US Food and Drug Administration, Japan's medical device agency, and the European Medicines Agency. In cardiology, imaging also plays an important role in the rapidly growing area of catheter ablation for atrial fibrillation (AF). The aim of the current study was to evaluate the feasibility and applicability of a GBCA-free protocol for the anatomical assessment of the heart for pulmonary vein isolation.
In a series of consecutive patients referred for catheter ablation of atrial fibrillation, MRI acquisition was performed using a GBCA-free protocol. The study was conducted according to the criteria set by the declaration of Helsinki and informed consent was obtained from all individual participants included in the study.
MRI was performed on 1.5 T scanners (Magnetom Avanto/Espree, Siemens, Erlangen, Germany). A 3D free-breathing respiratory navigator gated steady-state free precession sequence was performed. This approach minimized respiratory motion by acquiring data within a small gating window at a predefined respiratory position. MRI parameters were: TR 206.50 ms, TE 1.2 ms, flip angle 90°. The acquisition window and trigger delay were depended on the individual patient. The voxel size was 0.9 x 0.9 x 2.0 mm3. Slice thickness was 2 mm with 20% slice oversampling. MRI acquisition time was assessed from the start of the first scan to the end of the last scan and scan duration was assessed from the start to the end of the steady-state free precession sequence.
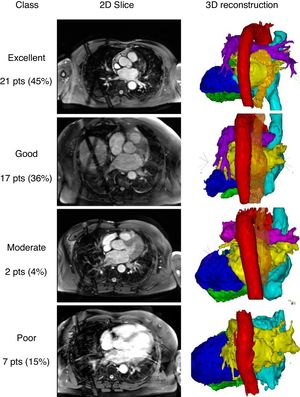
Segmentation and reconstruction were performed using CartoMerge software (Biosense Webster, Diamond Bar, United States). First, a threshold filter was set based on the image intensity to exclude voxels with a lower image intensity than the voxels of the relevant cardiac structure. Second, chambers of interest were tagged with points on their surface for the segmentation based on a region-growing algorithm. If the chambers were not separated correctly, manual processing to cut away irrelevant structures or create detectable boundaries was allowed. Finally, 3-dimensional surface reconstructions were performed. Qualitative assessment was performed based on the ease of segmentation and the quality of the reconstructed heart chambers to accurately represent the pulmonary vein anatomy and orientation up to the first bifurcation. Reconstructions were categorized as: excellent (without manual interaction); good (little manual interaction); moderate (extensive manual interaction); and poor (left atrium (LA) anatomy and pulmonary vein orientation could not be reconstructed). Representative examples of the categories are shown in figure 1. Segmentation of the esophagus was performed after cardiac segmentation due to its lower signal intensity.
Examples of the 2-dimensional (2D) slices and 3-dimensional (3D) reconstructions. Classifications: excellent (without manual interaction); good (little manual interaction), moderate (extensive manual interaction); poor (left atrium anatomy and pulmonary vein orientation could not be reconstructed). Red, aorta; yellow, left atrium; orange, esophagus; bright blue, right atrium; dark blue, left ventricle; green, right ventricle; pts, patients.
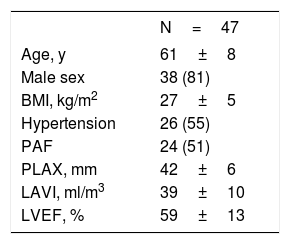
We included 47 patients in our study. The patients’ baseline and echocardiographic data are shown in table 1. Whole heart visualization including all chambers and the esophagus was possible in 40 patients. The acquisition time and scan duration was 25.8 (22.0-32.5) minutes and 8.3 (6.3-11.5) minutes, respectively. Image quality was adequate (excellent, good, and moderate) with all cardiac structures and segments in 85% of the patients. The esophagus was visible and could be segmented in 91% of the patients. No difference between scans with adequate or poor quality in any of the baseline or procedural data could be identified.
Baseline parameters
| N=47 | |
|---|---|
| Age, y | 61±8 |
| Male sex | 38 (81) |
| BMI, kg/m2 | 27±5 |
| Hypertension | 26 (55) |
| PAF | 24 (51) |
| PLAX, mm | 42±6 |
| LAVI, ml/m3 | 39±10 |
| LVEF, % | 59±13 |
BMI, body mass index; LAVI, left atrial volume indexed; LVEF, left ventricular ejection fraction; paroxysmal AF; PAF, paroxysmal atrial fibrillation; PLAX, left atrial size in parasternal long axis.
The data are expressed as No. (%) or mean±standard deviation.
The main findings of this study are: a) The GBCA-free acquisition protocol was feasible in all patients referred for catheter ablation of atrial fibrillation without complications. b) Anatomical assessment of the heart and in particular of the LA for electrophysiological procedures using a contrast-free native MRI protocol is usable in 85% of the patients. c) The course of the esophagus was visible in 91% of the patients.
In addition to the potential toxicity of GBCA, other rare complications such as allergic reactions have been documented.2 With a GBCA-free acquisition protocol, these complications can be prevented. Furthermore, no venous access is required to enable contrast injection. In addition to the potential reduction in complications, right-sided cavities can be visualized, which facilitates catheter placement and transseptal puncture. To visualize the course and the width of the esophagus, electroanatomical mapping using the ablation catheter3 or radiological visualization of the swallowed barium paste has been reported.4 Preprocedural, noninvasive assessment is possible using for instance a 3-dimensional late gadolinium enhanced MRI sequence, which allows the visualization of the course and the width of the esophagus in addition to the fibrosis in the LA.5 In contrast to these complex or invasive strategies, the GBCA-free MRI approach has the potential to be used as simplified preprocedural assessment of the heart and the surrounding structures. Ideal candidates might be patients who are able to lie supine comfortably and who have a regular breathing pattern and RR intervals. However, it remains to be determined whether the visualization of the esophagus and its location with regards to the LA and the ablation set might prevent complications such as atrioesophageal fistula.
In conclusion, anatomical assessment of the entire heart and in particular of the LA for electrophysiological procedures using a contrast-free native MRI protocol is sufficient in 85% of the patients. Despite the relatively long acquisition duration, the protocol is especially useful for patients with renal insufficiency or to visualize the course of the esophagus in addition to the heart anatomy.
Conflicts Of InterestM. Kühne is proctor for Medtronic, speakers bureau for Boston Scientific, St Jude Medical, and Biotronik. C. Sticherling is advisory board of Medtronic and Biotronik, received educational grants from Biotronik and research grant from Biosense Webster, outside the submitted work.