Aortic regurgitation (AR) of at least moderate severity is a frequent complication in patients with a long-term continuous flow left ventricular assist device (LVAD) (affecting 25%-30% of patients in the first year). However, its prevention and management are sources of controversy, given the limited evidence available. In addition, its clinical presentation can be atypical and may delay the diagnosis of this potentially life-threatening complication.1
We present the case of a 72-year-old man with a mechanical mitral prosthesis after native valve endocarditis in 2003. The patient had an extensive anterior myocardial infarction due to a septic embolism that progressed to severe left ventricular dysfunction. Optimal treatment was implemented and he had good functional status until he progressively deteriorated about 2 years ago, developing dyspnea on minimal exertion and needing several hospitalizations that sometimes required the use of inotropic agents. Workup was begun to evaluate the possible use of advanced treatments. In September 2018, a HeartMate 3 long-term LVAD (Abbott; Santa Clara, California, United States) was implanted as destination therapy. Echocardiography showed absence of aortic valve opening and minimal AR.
About 5 months later, the patient was admitted due to deterioration in his overall condition. He had no dyspnea or any other clinical signs or symptoms of heart failure and was afebrile, hemodynamically stable, and without congestive signs. Mild cutaneous erythema at the exit site of the percutaneous LVAD lead was treated with empirical antibiotics. Preantibiotic culture results were negative. Blood tests revealed creatinine 1.66mg/dL, slight transaminase elevation with total bilirubin 4.1mg/dL, a normal complete blood count, and an INR of 4.8. Although hemolysis data were not obtained, blood cultures and serological tests for hepatotropic viruses were negative and abdominal ultrasound findings were unremarkable. After 48hours, the patient exhibited a decreased and fluctuating level of consciousness and was tachypneic with signs of poor distal perfusion, even with 95% arterial blood oxygen saturation and without focal neurological deficits. Cranial computed tomography was unremarkable. Blood tests revealed compensated metabolic acidosis, with lactate 3.5 mmol/L, and further deterioration in liver function tests. The relevant device parameters were 3200rpm and flow 5 L/min but the mixed venous oxygen saturation was 47%, indicating insufficient cardiac output despite adequate flow.
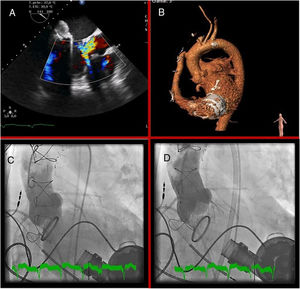
Despite cardiogenic shock, transthoracic echocardiography revealed normal right ventricular function and severe AR, which was confirmed on transesophageal echocardiography (figure 1A); there were no signs of endocarditis. We decided to perform emergency transcatheter aortic valve implantation with an EVOLUT device (Medtronic; Minneapolis, Minnesota, United States) (figure 1B–D). The patient's subsequent clinical course and blood tests were favorable and he was discharged with good functional status, which continued until at least 6 months after the implantation.
The HeartMate 3 long-term LVAD is a continuous flow device that can be used as destination therapy. Experience with this type of LVAD is slight. Accordingly, early identification of complications can be a challenge.
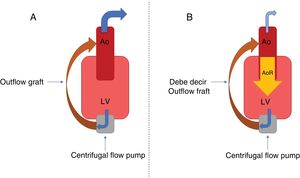
Although technological advances have reduced device thrombosis and embolic events,2 AR occurrence is still common. Its development is related to a fall in the percentage of valve opening (which favors commissure fusion and tissue remodeling) and the effects of the nonpulsatile flow on the aortic root, which is subjected to hemodynamic stress during the entire cardiac cycle. The end result is valve failure and the establishment of an inefficient cycle—LVAD-aorta-left ventricle-LVAD (figure 2)—with decreased peripheral perfusion and recurrent symptoms of heart failure. Various patient-dependent factors predispose its development: small body surface, female sex, and advanced age. Other factors are the age of the LVAD, a persistently closed valve, excessive left ventricular unloading, and the anastomosis angle from the outflow graft. No significant differences have been found among devices.1
Diagnosis can be complicated due to a sometimes atypical and deceptive clinical presentation. The acoustic window is usually limited and traditional echocardiographic parameters underestimate the severity of the regurgitation, given that the AR continues throughout the entire cardiac cycle, increasing the regurgitant volume. New parameters have been described for its quantification: the systolic-to-diastolic peak velocity ratio and the outflow cannula diastolic acceleration.3
The management of this complication is challenging because surgical risk can sometimes be prohibitive in these patients. Accordingly, percutaneous devices are becoming a therapeutic option, despite limited evidence. Both transcatheter aortic prostheses4 and percutaneous occlusion devices5 have been used. Both approaches achieve favorable short-term hemodynamic outcomes.
For this patient, transcatheter aortic valve implantation was chosen due to the experience of our center and the availability of the technique.
In conclusion, the atypical clinical presentation, early development of valve disease, and comorbidity of this patient are another example of the challenge posed by LVAD therapy and of the importance of a multidisciplinary approach.
CONFLICTS OF INTERESTC. Morís de la Tassa is a proctor for Medtronic.