Left ventricular assist devices (LVAD) are a success story in the treatment of patients with advanced heart failure, but their use is limited by the incidence of adverse events. One of the more serious complications is device thrombosis. With third generation devices, the incidence rate for thrombosis is between 1.35 and 0.5/100 patients/mo, and thrombosis is more common within the pump. The incidence of intrapump thrombosis has been reduced by technological advances1; however, LVAD flow can also be obstructed, albeit less frequently, by torsion, thrombosis, or stenosis affecting the outflow graft (4% of thrombosis events).2
We present the case of a 72-year-old male patient with advanced heart failure in INTERMACS profile 3 who received a HeartWare HVAD System (Medtronic, United States) as destination therapy. The LVAD was implanted via left lateral thoracotomy and upper ministernotomy. The outflow graft was anastomosed in the ascending aorta. The patient was anticoagulated with sodium heparin in the first 24hours after implantation and was subsequently placed on oral anticoagulation therapy with acenocoumarol and antiplatelet therapy with 150mg/d aspirin. The HeartWare HVAD parameters at discharge were as follows: speed, 2700rpm; power, 3.6W; and estimated flow, 3.9 L/min. Echocardiography confirmed intermittent opening of the aortic valve.
The patient's immediate clinical course was favorable. However, after the fourth month, he began to have events related to hemocompatibility. There were 2 episodes of severe anemia secondary to occult gastrointestinal bleeding that required temporary suspension of antiplatelet and anticoagulation therapies, management to achieve an INR of 2-2.5, and initiation of chronic treatment with slow-release octreotide. The patient also had a transient ischemic attack in the right middle cerebral artery.
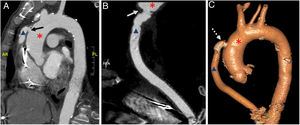
LVAD follow-up revealed a progressive decline in estimated flow but with stable power despite blood volume optimization. This was accompanied by a decline in flow pulsatility and hemolysis data showing progressive increases in lactate dehydrogenase levels and haptoglobin metabolism, despite good INR control except during the bleeding episodes. Echocardiography revealed evidence of insufficient left ventricular emptying: aortic valve opening in all heartbeats, moderate mitral regurgitation, and displacement of the interventricular septum to the right. Thoracic computed tomography revealed stenosis of the anastomosis between the outflow graft and the ascending aorta (figure 1A,B). The outflow graft was at a 90° angle to the aorta and there was no evidence of thrombotic material, thus indicating torsion of the graft. Given the evidence indicating hemodynamically significant stenosis, the decision was taken to intervene percutaneously.
A and B, Thoracic computed tomography showing the stenosis (solid arrows) located at the level of the anastomosis of the outflow graft (triangle) with the ascending aorta (asterisk). C, Postprocedure computed tomography 3-dimensional reconstruction showing the implanted stent (dashed arrow).
The procedure was carried out in the catheterization laboratory using a retrograde approach via the outflow graft. The patient was previously fitted with a SENTINAL cerebral protection device (Boston Scientific, United States). A 10/30mm Formula stent (Cook Medical, United States) was placed in the outflow graft stenosis, followed by dilation with a 12mm balloon (figure 1C).
Hemodynamic data and LVAD parameters during the procedure are shown in table 1. Postprocedure echocardiography showed complete closure of the aortic valve, disappearance of the mitral regurgitation, and midline positioning of the interventricular septum, indicating appropriate decompression of the left ventricle by the LVAD. After the procedure, the patient was placed on dual antiplatelet therapy with 100mg/d aspirin and 75mg/d clopidogrel and was anticoagulated to a target INR of 2–3. Lactate dehydrogenase levels normalized, and the patient has had no further gastrointestinal bleeding events during 6 months of follow-up.
LVAD parameters and hemodynamic variables before and after dilation of the outflow graft
| Hemodynamic measurements upstream of the stenosis | Hemodynamic measurements in the ascending aorta | Mean gradient, mmHg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP, mmHg | DBP, mmHg | MBP, mmHg | SBP, mmHg | DBP, mmHg | MBP, mmHg | ||||||||
| Pre-PI | Post-PI | Pre-PI | Post-PI | Pre-PI | Post-PI | Pre-PI | Post-PI | Pre-PI | Post-PI | Pre-PI | Post-PI | Pre-PI | Post-PI |
| 200 | 124 | 130 | 105 | 155 | 110 | 83 | 85 | 41 | 78 | 60 | 81 | 95 | 29 |
| LVAD data | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Speed, rpm | Power, W | Flow, L/min | Pulsatility, L/min | ||||||||
| Baseline | Pre-PI | Post-PI | Baseline | Pre-PI | Post-PI | Baseline | Pre-PI | Post-PI | Baseline | Pre-PI | Post-PI |
| 2700 | 3200 | 3200 | 3.6 | 6.2 | 6.3 | 3.9 | 2.3 | 4.4 | 2.5 | 1 | 3.5 |
DBP, diastolic blood pressure; LVAD, left ventricular assist device; MBP, mean blood pressure; PI, percutaneous intervention; rpm, revolutions per minute; SBP, systolic blood pressure.
This patient's case illustrates an uncommon problem that highlights 2 issues. The first is the importance of investigating and treating chronic hemolysis in patients with an LVAD, a situation that reduces survival and increases the rate of adverse events.3 The second issue is the influence of the surgical method on the prevention of adverse events. In the case presented here, the sharp angle of the anastomosis could have caused the hydrodynamic forces and turbulence to progressively increase the flow obstruction and produce progressive torsion of the outflow graft.4 While the choice of approach will depend on the size and location of the stenosis, percutaneous treatment is useful and safe in selected patients.
FUNDINGThis study received funding from the European Regional Development Fund (ERDF) through the Biomedical Research Networking Center on Cardiovascular Diseases (CIBERCV), Instituto de Salud Carlos III, Madrid, Spain.
We thank Dr Concepción Crespo García and Dr Susana Otero Muinelo of the Radiogiagnosis Service at A Coruña University Hospital, Instituto de Investigaciones Biomédicas de A Coruña (INIBIC).