Truncating titin variants (TTNtv) are the main genetic cause of dilated cardiomyopathy (DCM).1 These variants have been associated with a mild and treatable form of DCM2 (the need for a ‘second hit’ such as chemotherapy or alcohol abuse has been even suggested),3 but also with an increased risk of arrhythmias/sudden death.4,5 The latter has aroused concerns about a lower threshold for defibrillator implantation, as practiced in other genetic forms of DCM.
The titin (TTN) gene encodes 364 exons that undergo alternative splicing to produce different isoforms. In the adult myocardium, 2 major TTN isoforms, N2BA and N2B, are mainly expressed. Most of truncating TTN variants affect these cardiac TTN isoforms, being predominantly located at the A-band.
We present a retrospective single referral-center cohort study exploring the phenotype and prognosis of TTNtv-DCM patients compared with a well-defined control group composed of carriers of variants in other DCM-related genes.
We selected 129 adult patients with DCM/hypokinetic nondilated cardiomyopathy and genetic testing. Of these, 47 tested positive (ie, pathogenic or likely pathogenic variant according to the American College of Medical Genetics and Genomics guidelines), 56 negative, and 26 inconclusive. A total of 2 double pathogenic variant-carriers were excluded, with a final result of 45 patients: 19 TTNtv-probands, 4 phenotype-positive relatives, and 22 non-TTN probands.
For comparisons, the sample was divided into TTNtv patients (probands and phenotype-positive relatives, n=23) and non-TTN patients (n=22).
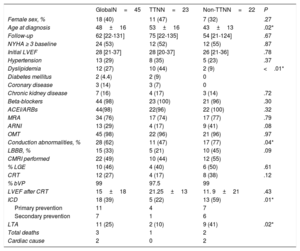
Demographic and clinical characteristics are shown in table 1.
Demographic and clinical characteristics
| GlobalN=45 | TTNN=23 | Non-TTNN=22 | P | |
|---|---|---|---|---|
| Female sex, % | 18 (40) | 11 (47) | 7 (32) | .27 |
| Age at diagnosis | 48±16 | 53±16 | 43±13 | .02* |
| Follow-up | 62 [22-131] | 75 [22-135] | 54 [21-124] | .67 |
| NYHA ≥ 3 baseline | 24 (53) | 12 (52) | 12 (55) | .87 |
| Initial LVEF | 28 [21-37] | 28 [20-37] | 26 [21-36] | .78 |
| Hypertension | 13 (29) | 8 (35) | 5 (23) | .37 |
| Dyslipidemia | 12 (27) | 10 (44) | 2 (9) | <.01* |
| Diabetes mellitus | 2 (4.4) | 2 (9) | 0 | |
| Coronary disease | 3 (14) | 3 (7) | 0 | |
| Chronic kidney disease | 7 (16) | 4 (17) | 3 (14) | .72 |
| Beta-blockers | 44 (98) | 23 (100) | 21 (96) | .30 |
| ACEI/ARBs | 44(98) | 22(96) | 22 (100) | .32 |
| MRA | 34 (76) | 17 (74) | 17 (77) | .79 |
| ARNI | 13 (29) | 4 (17) | 9 (41) | .08 |
| OMT | 45 (98) | 22 (96) | 21 (96) | .97 |
| Conduction abnormalities, % | 28 (62) | 11 (47) | 17 (77) | .04* |
| LBBB, % | 15 (33) | 5 (21) | 10 (45) | .09 |
| CMRI performed | 22 (49) | 10 (44) | 12 (55) | |
| % LGE | 10 (46) | 4 (40) | 6 (50) | .61 |
| CRT | 12 (27) | 4 (17) | 8 (38) | .12 |
| % bVP | 99 | 97.5 | 99 | |
| LVEF after CRT | 15±18 | 21.25±13 | 11. 9±21 | .43 |
| ICD | 18 (39) | 5 (22) | 13 (59) | .01* |
| Primary prevention | 11 | 4 | 7 | |
| Secondary prevention | 7 | 1 | 6 | |
| LTA | 11 (25) | 2 (10) | 9 (41) | .02* |
| Total deaths | 3 | 1 | 2 | |
| Cardiac cause | 2 | 0 | 2 |
ACEI, angiotensin converting enzyme inhibitor; ARBs, angiotensin receptor blockers; ARNI, antiotensin II receptor blocker neprilysin inhibitor; bVP, biventricular pacing; CMRI, cardiac magnetic resonance imaging; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LBBB: left bundle branch block; LGE, late gadolinium enhancement; LTA, life-threatening arrhythmias; LVEF, left ventricle ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA; New York Heart Association; OMT, optimal medical therapies; TTN, titin.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
In terms of left ventricular ejection fraction (LVEF) after diagnosis and treatment, 2 possible scenarios were established: a) favorable behavior, in patients with a significant LVEF increase (at least 10% increase and LVEF >30% after improvement was achieved, or patients with their first 2 LVEF values above 40%); and b) unfavorable behavior, if no favorable behavior as described was present.
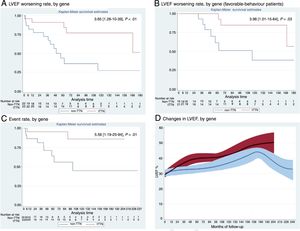
The primary endpoint was LVEF worsening. Time of worsening was defined as LVEF<40%, or at least 10% below the best value of each favorable-behavior patient. In unfavorable-behavior patients, time of worsening was established at the second transthoracic echocardiogram. During a median of 62 months of follow-up, non-TTN patients showed a 3.6-fold risk of LVEF worsening compared with TTNtv patients (1.28-10.39, P<.01; figure 1A). When we analyzed favorable patients only (82%; 91% TTNtv vs 72% non-TTN), the differences persisted: non-TTN patients showed a 3.98-fold risk of worsening (1.01-15.64, P=.03; figure 1B). Time to LVEF worsening was clearly different in both groups: at 5 years of follow-up, LVEF was maintained in all favorable-behavior TTNtv patients and in only 63% of non-TTN (favorable) patients.
Outcomes in TTNtv dilated cardiomyopathy. TTNtv in red, non-TTN in blue. Survival analysis of: A: LVEF worsening; B: LVEF worsening in favorable-behavior patients, and C: major events (death, heart transplant, or left ventricular assist device implantation. D: Changes in LVEF, by gene. For LVEF worsening analysis, outliers were excluded, delimiting follow-up in 180 months. LVEF, left ventricular ejection fraction; TTNtv, truncating titin variants.
Secondary endpoints included: a) composite clinical endpoint: non-TTN patients showed a 5.56-fold risk of death, heart transplant, or left ventricular assist device (1.19-25.94, P=.01; figure 1C); and b) the presence of a significant increase in LVEF after diagnosis and treatment. LVEF improved in most patients (73% globally; 83% TTNtv vs 64% non-TTN, P=.15). LVEF trends were represented by Loess curves for gene subgroups and were analyzed using a linear mixed model for repeated measurements, with a statistically significant difference (P <.05) between them (figure 1D).
Unlike previous works, we found no significant differences in LVEF recovery between TTNtv and non-TTN patients. Although this may be due to the sample size, our more restrictive definition could also have played a role. We established LVEF improvement as an exclusively structural endpoint, and only considered increases of more than 10% if the final LVEF was above 30%. In our opinion, this 30% threshold is necessary because some LVEF changes in the severely depressed area might not be clinically relevant.
Moreover, we did not compare TTNtv-DCM with idiopathic DCM. This hodgepodge term could distort results when aiming to correlate a particular genotype with phenotypic manifestations. A comparison to a well-defined control group, comprising other genetic-confirmed DCM cases, have been preferred.
Equally, we did not observe that TTNtv-DCM patients had a milder initial form: our patients had similar LVEF (28 in TTNtv vs 26) and New York Heart Association functional class at diagnosis (52% of patients above New York Heart Association II vs 55%, respectively). However, we did observe that, under optimal medical treatment, patients with TTNtv-DCM were significantly more likely to maintain the LVEF recovery achieved, which, to the best of our knowledge, has not been previously described.
A compensated-state myocardium hypothesis, intolerant to further stress, has been proposed, relating the etiopathogenesis of TTNtv-DCM to nonsense-mediated decay.6 It also seems plausible that the profile of TTN isoform expression changes in the myocardium under altered charge conditions. The loss of an allele of the stiffer N2B isoform might affect the expression ratio and, as a consequence, myocardium compliance properties, in the same way as medical treatment might be a key factor to prevent TTNtv myocardium from damage.
All these data depict DCM associated with TTNtv not as a milder form of the disease, but as a more malleable entity, with a more sustained LVEF response and less severe progression in terms of major cardiac events. These results, along with previous reported outcomes in TTNtv-DCM, support the idea of a genetic-based management, with particular thresholds for therapies and devices, a less close follow-up and, last but not least, a reassuring prognostic message to these TTNtv-DCM patients.
We gratefully acknowledge the participation of every family member included in this research and the work of our nurses: Begoña Navarro and Natalia Maganto.